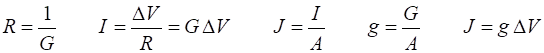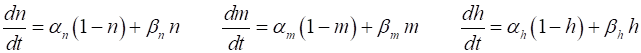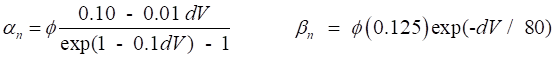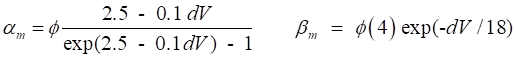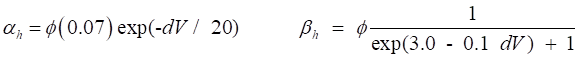|
HODGKIN – HUXLEY MODEL FOR MEMBRANE CURRENTS AND ACTION
POTENTIALS Ian Cooper Email: matlabvisualphysics@gmail.com |
|
MATLAB npHHA.m The membrane potential as a function of time of a neuron is calculated using the Hodgkin – Huxley model. A variety of current injection stimuli can be used to view the time evolution of the membrane potential. The stimulus is selected using the variable flagJ. The amplitude of the stimulus is set by the variable J0 where J0 is the maximum current density [A.cm-2] injected into the neuron. flagJ = 1 single square current pulse flagJ = 2 constant current injection: step function flagJ = 3 double current pulse flagJ = 4 series of current pulses flagJ = 5 sinusoidal current stimulus flagJ = 6 series of current pulses with noise added to each pulse The model parameters are set within each switch/case section. The differential equations are solved using a finite difference method. bp_neuron_01bb.m Data from npHHA.m to plot the spike firing rate against the amplitude of a constant current injection (figure 15). |
|
HODGKIN – HUXLEY MODEL The core mathematical framework
for modern biophysically based neural modelling was developed around 1950 by
Alan Hodgkin and Andrew Huxley. They carried out a series of elegant
electrophysiological experiments on squid giant neurons which have
extraordinarily large diameters (~ 0.5 mm). Hodgkin and Huxley
systematically demonstrated how the macroscopic ionic currents in the squid
giant axon could be understood in terms of changes in Na+ and K+
conductances in the axon membrane. Based on a series of voltage-clamp
experiments, they developed a detailed mathematical model of the
voltage-dependent and time-dependent properties of the Na+ and K+
conductances. Their model accurately reproduces the key biophysical
properties of the action potential. For this outstanding achievement, Hodgkin
and Huxley were awarded the 1963 Nobel Prize in Physiology and Medicine. In biophysically based neural modelling, the electrical
properties of a neuron are represented in terms of an electrical equivalent
circuit. Capacitors are used to model the charge storage capacity of the
membranes (a
semipermeable cell membrane separates the interior of the cell from the
extracellular liquid and acts as a capacitor). Resistors are used to model the various types of
ion channels embedded in the membrane, and batteries are used to represent
the electrochemical potentials established by differing intracellular and
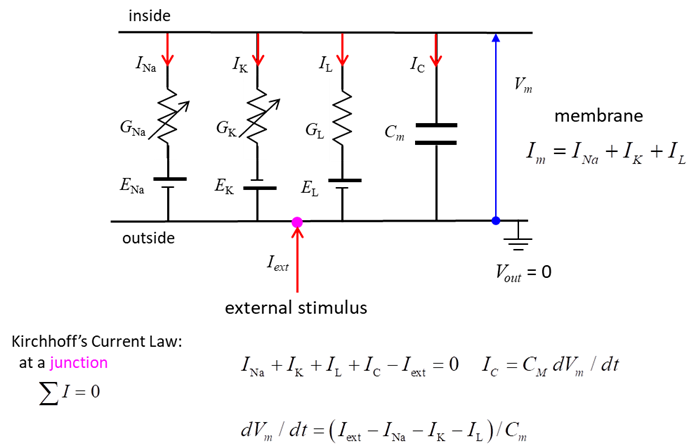
extracellular ion concentrations. Figure 1 shows the equivalent circuit used
by Hodgkin and Huxley in modelling a segment of squid giant axon. The current across the membrane has
two major components, one associated with the membrane capacitance and one
associated with the flow of ions through resistive membrane channels. They found three different types of
ion currents: Na+, K+, and a leak current that consists
mainly of Cl- ions. The flow of ions through a cell membrane of a neuron are
controlled by special voltage dependent ion channels: Na+ ion
channel, K+ ion channel and a leak ion channel for all other ions.
The neuron can be stimulated by an external current Iext injected into the interior of
the neuron.
Fig.1. Electrical
equivalent circuit for a short segment of squid giant axon. Capacitor
(capacitance Cm of the cell membrane);
Variable resistors (voltage-dependent Na+ and K+ conductances GNa, GK ); fixed resistor (voltage-independent leakage conductance
GL); Batteries (reversal
potentials Na+, K+ , leakage: ENa, EK, EL); Membrane potential V = Vm = Vin - Vout;
External stimulus Iext; Current directions
(arrows: Iext outside ® inside (I < 0), INa,
IK
and IL inside ® outside (I > 0). Conductance
G, resistance R ® G = 1 / R. Electrical activity in neurons is sustained
and propagated by ion currents through neuron membranes as shown in figure 1.
Most of these transmembrane currents involve four
ionic species: sodium Na+, potassium K+, calcium Ca2+
and chloride (Cl-). The concentrations
of these ions are different on the inside and outside of a cell. This creates
the electrochemical gradients which are the major driving forces of neural
activity. The extracellular
medium has high concentration of Na+ and Cl-
and a relatively high concentration of Ca2+. The intracellular medium has high concentration of K+
and negatively charged large molecules A-. The cell membrane has
large protein molecules forming ion channels through
which ions (but not A-) can flow according to their
electrochemical gradients. The concentration asymmetry is maintained
through ·
Passive redistribution: The impermeable anions A-
attract more K+ into the cell and repel more Cl-
out of the cell. ·
Active transport: Ions are pumped in and out of
the cell by ionic pumps. For example, the Na+/K+ pump,
which pumps out three Na+ ions for every two K+ ions
pumped. In the
Hodgkin – Huxley model only the movement of the sodium, potassium ions
are considered, all other ions are considered as part of the leak current. A mathematical analysis of the equivalent RC circuit
for the neuron as shown in figure 1 is outlined by the following equations. Membrane potential difference measured w.r.t. Vout
= 0
(1)
Vm = Vin
– Vout Capacitive current: rate
of change of charge Q at the membrane surface (2)
Charge stored on surface of membrane (3)
Differentiating Q w.r.t. t at a fixed position x0
(4)
Membrane current due to movement of ions
(5)
Kirchhoff’s current law (conservation of
charge)
(6)
The fundamental differential equation
relating the change in membrane potential to the currents through the
membrane for a small segment of the membrane:
(7) Equation 7 is better expressed in terms of current
density J
rather than current I. Dividing equation 9 by an area
A,
equation 7 becomes
(8) where Electrical potential (voltage) DV, current I, resistance R and conductance G are related by the equations
In applying these relationships to ion channels, the equilibrium
(reversal) potential for each ion type also needs to be taken into account.
This is the potential at which the net ionic current flowing across the
membrane would be zero for a given ion species. The reversal potentials are
represented by the batteries in figure 1. Hence,
(9)
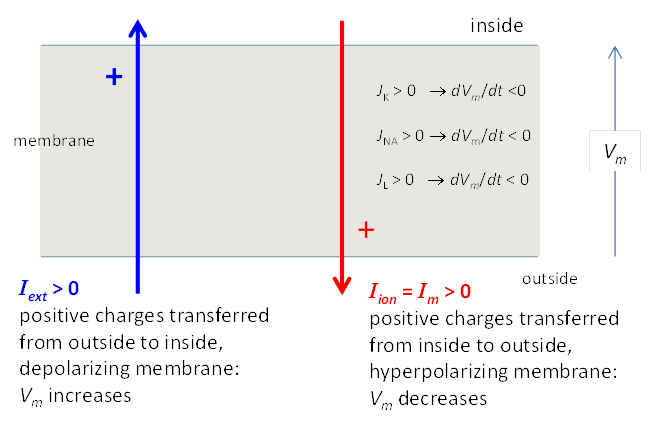
Fig. 3. Sign convention for currents. A
positive external current Iext (outside to
inside) will tend
to depolarize the cell (i.e., make Vm more positive) while a positive ionic current Iion will tend to hyperpolarize the cell (i.e., make V = Vm more negative). In a simple model, the Na+ and K+ ions are
considered to flow through ion channels where a series of gates determine the
conductance of the ion channel. The macroscopic conductances of the Hodgkin
& Huxley model arise from the combined effects of a large number of
microscopic ion channels embedded in the membrane. Each individual ion
channel can be thought of as containing one or more physical gates that
regulate the flow of ions through the channel. The variation in g values is determined by the set of gate variables n,
m and h. An activation gate ® conductance increases with depolarization An inactivation gate ® conductance decreases with depolarization The Na+
channel is controlled by 3m activation gates and 1h inactivation gate The K+ channel is controlled by 4n activation gates From experimental data, the conductances are assumed
to described by the following functions
(10) The value of the conductance depends upon the
membrane voltage Vm because the values of n,
m
and h
depend on time, their previous value at an earlier time and the membrane
potential. The resting membrane potential is given by the symbols Vrest
or Vr. The rates of change of the gate variables are
described by the equations
(11) where the
If there is no external stimulus J0
= 0 and Vm = Vr then Jm = 0 and Vm does not change with time t
as dVm/dt = 0. A stimulus as a result of a current injection into
the axon results in the membrane potential either increasing above or
decreasing below the resting membrane potential. The charge per unit area Q deposited into
intracellular region by the external stimulus Jext is given by
Fig.
4. A strong stimulus to the
neuron will evoke an action potential. The value of the Hodgkin – Huxley parameters used in the modelling are % FIXED PARAMETERS
==================================================== sf =
1e3;
% conversion of V
to mV VR = -65e-3; % resting voltage [V] Vr =
VR*1e3;
% resting voltage
[mV] VNa =
50e-3;
% reversal voltage
for Na+ [V] VK =
-77e-3;
% reversal voltage
for K+ [V] Cm = 1e-6;
% membrane
capacitance/area [F.cm^-2] gKmax
= 36e-3; % K+ conductance [S.cm^-2] gNamax
= 120e-3; % Na+ conductance [S.cm.-2)] gLmax
= 0.3e-3; % max leakage conductance [S.cm-2] T =
20;
% temperature [20 deg C] NUMERICAL SOLUTIONS OF THE HODGKIN – HUXLEY MODEL The Script npHHA.m is
used to solve equation 8 numerically using a finite difference method to
approximate the derivatives. In this subsection we study the dynamics of the
Hodgkin-Huxley model for different types of input which are specified by the
variable flagJ. Single current pulse flagJ = 1 Default input parameters case 1 % Single current pulse % Amplitude of pulse [100e-6 A.cm-2] J0
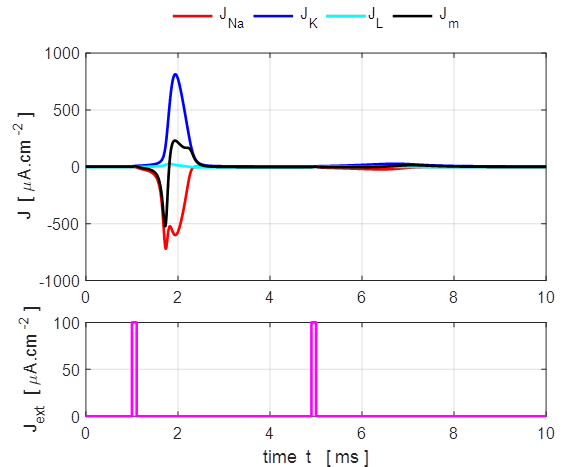
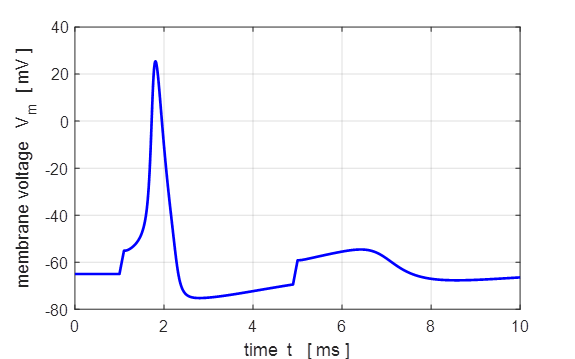
= 100e-6; % Simulation time [5.0e-3 s] tMax = 5e-3; % Time stimulus applied [0.5e-3 s] tStart = 0.5e-3; % Pulse duration: ON time [0.1e-3 s] tON = 0.1e-3; % Number of grid points [8001] num = 8001; If the stimulus is strong enough (sufficient charge
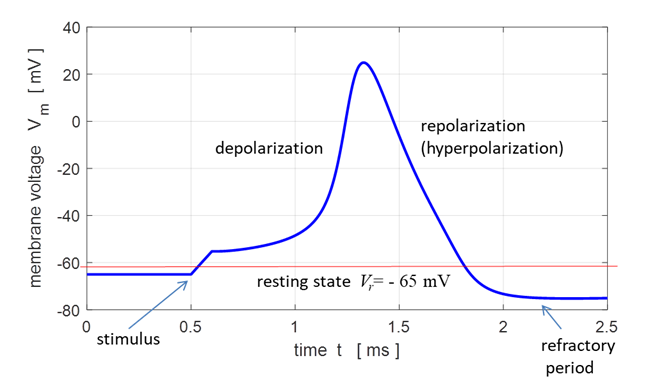
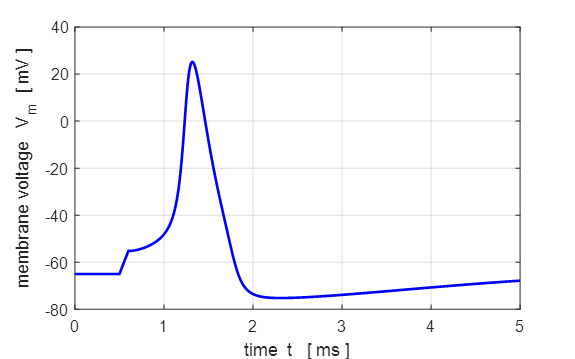
injected into the neuron) an action potential will be generated as shown in
figure 5.
Fig. 5. Action
potential produced by an external current pulse. The time course of the
membrane shows the action potential (positive peak) followed by a relative
refractory period where the potential is below the resting potential.
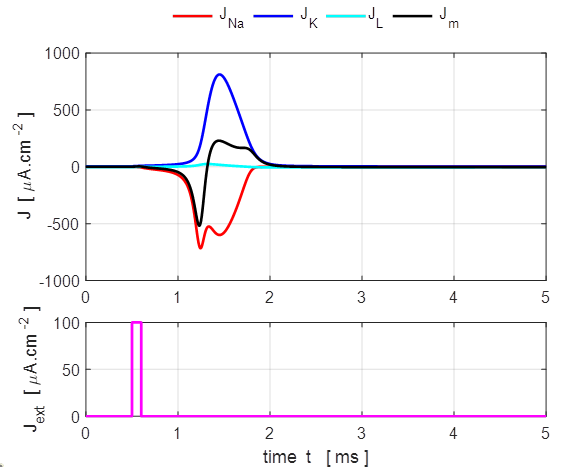
Fig. 6.
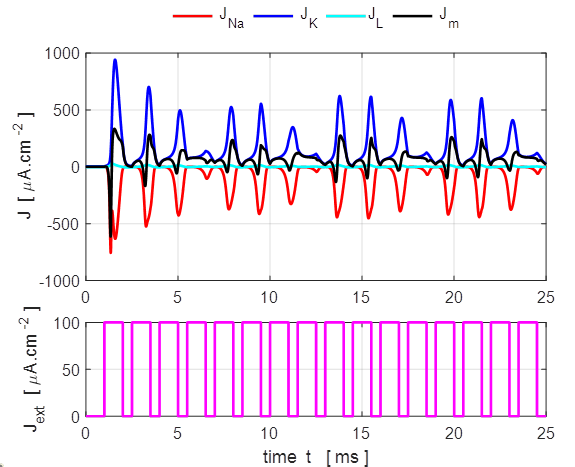
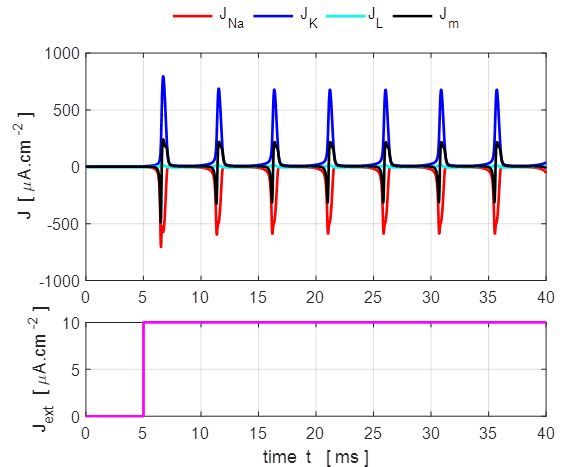
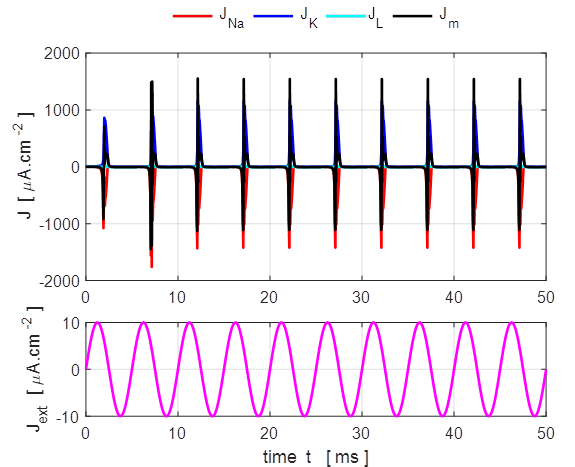
Only a very small current pulse is required to
dramatically change the conductances of the membrane to produce large K+
and Na+ currents. The potassium current is positive as the K+
ions move from inside to the outside of the cell whereas the sodium current
is negative as Na+ ions move into the cell across the membrane.
The Na+ and K+ currents are nearly balanced throughout
most of the duration of the action potential which lasts about 1 ms. The JNa curve has an extra wiggle around
t = 1.3 ms
caused by the rapidly changing voltage while the conductance gNa varies smoothly (figure 7).
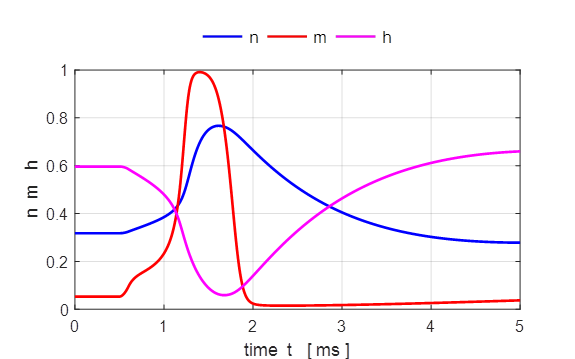
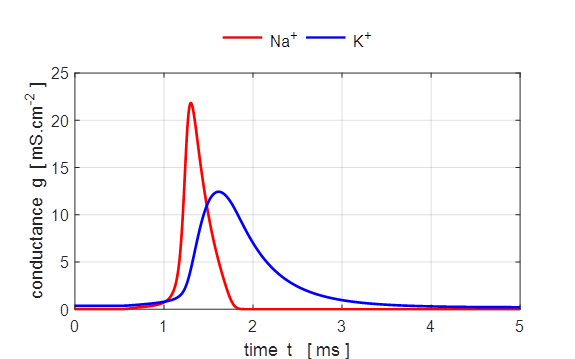
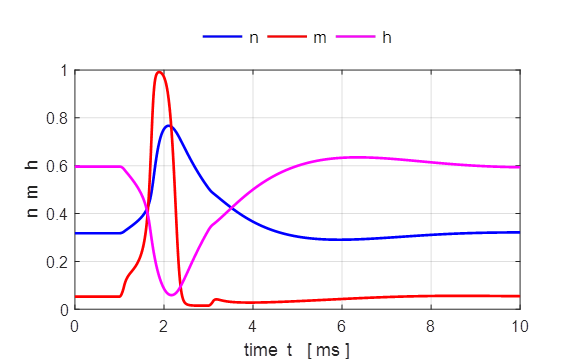
Fig. 7. The gate variables n, m and h and the conductance for
potassium and sodium as functions of time. Both the conductances for the Na+
and K+ ions vary smoothly. The rise in the sodium conductance and
fall occur more rapidly for Na+ than for K+ mainly due
to the behaviour of the gate variables m and h. We see that m and n increase with increasing Vm whereas h decreases. Thus, if some
external input causes the membrane voltage to rise, the conductance of sodium
channels increases due to increasing m. As a result, positive
sodium ions flow into the cell and raise the membrane potential even further.
If this positive feedback is large enough, an action potential is initiated.
At high values of Vm, the sodium conductance
is shut off due to the factor h. The time constant for h is always larger than m. Thus the variable h which closes the channels
reacts more slowly to the voltage increase than the variable m which opens the channel.
On a similar slow time scale, the potassium K+ current sets in. Since it is a
current in outward direction, it lowers the potential. The overall effect of
the sodium and potassium currents is a short action potential followed by a negative
overshoot.
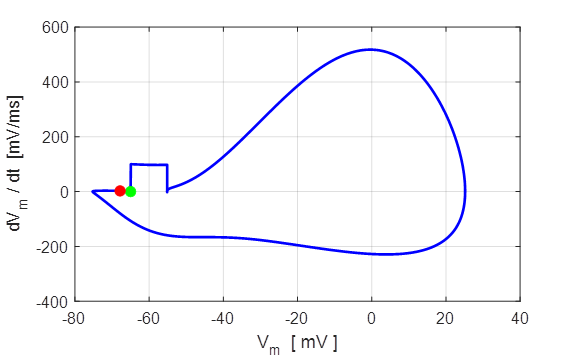
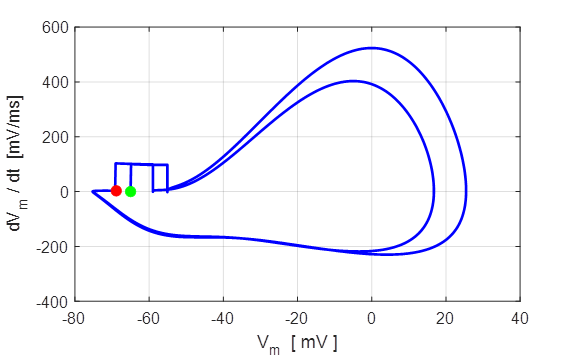
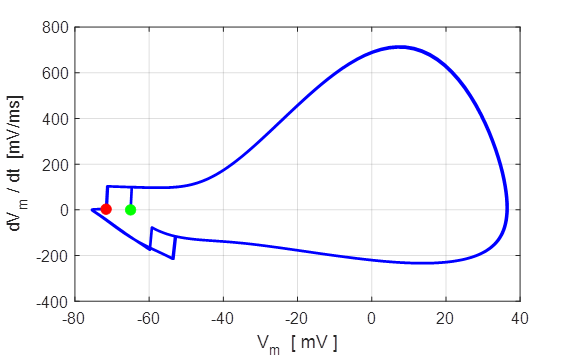
Fig. 8. Phase portrait plot. The
membrane potential which equals the rest potential is a stable equilibrium
point.
Green dot start (t = 0) and the red dot is
finish of the simulation.
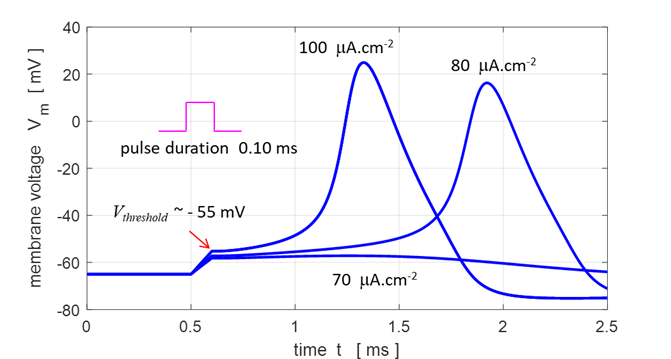
Fig.
9. Membrane responses to
three different external stimuli for a square pulse of duration 0.10 ms. J0 = 70
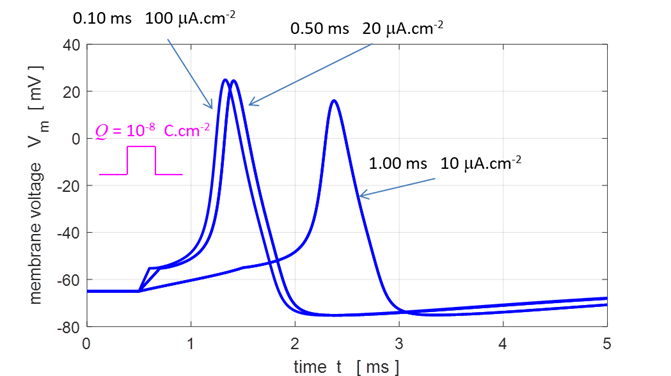
Fig.
10. The duration and height
of the single square pulse is varied such that the charge injected is
constant Q
= 1.00x10-8 C.cm-2. The pulse with the greatest amplitude
and shortest duration produces the action potential which rises most rapidly
and with the greatest depolarization.
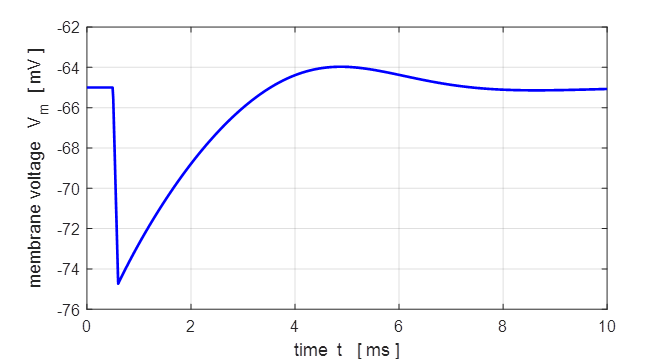
Fig.
11. A small negative pulse
(duration 0.10 ms and height -100 Dual Current
Pulses flagJ
= 3 We stimulate the Hodgkin-Huxley model by an initial
current pulse that is sufficiently strong to excite a spike and a second
current pulse of the same amplitude as the The response of the membrane potential of two
successive square pulses of duration 0.1 ms and
amplitude 100 Default input parameters case 3 % Double current pulse stimulus % Amplitude of pulses [100e-6 A.cm-2] J0
= 100e-6; % Simulation time [2.5e-3 s] tMax = 10e-3; % Pulse duration: ON time [0.1e-3 s] tON = 0.1e-3; % time pulse #1 ON [1.0e-3 s] / time pulse #2 ON [5.0e-3 s] t1
= 1.0e-3; t2
= 5.0e-3; % Number of grid points [8001] num = 8001; If the time interval between the two pulses is
greater than 4.0 ms (t1 = 1.0 ms
and t2 > 5.0 ms) then two spikes are generated
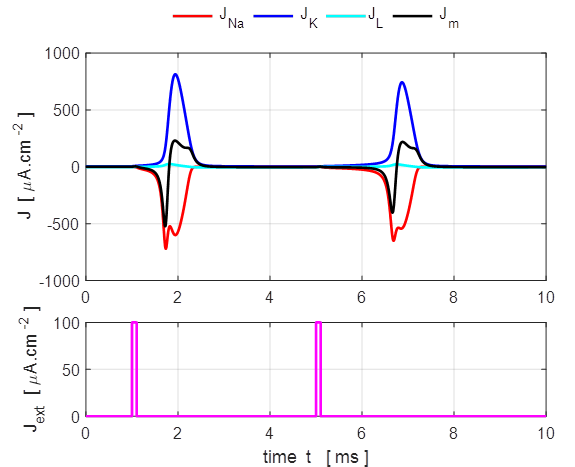
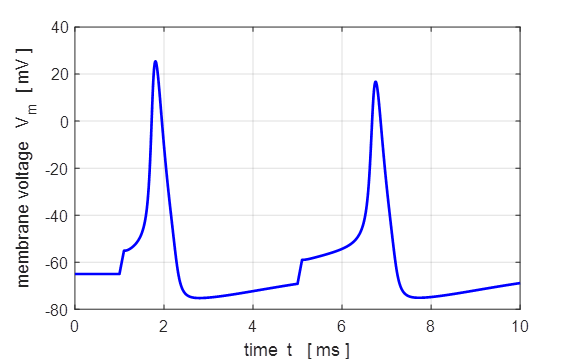
(figure 12A). When the time interval between pulses is set to 3.9 ms (t1 = 1.0 ms and t2 = 4.9 ms), a second spike is not produced (figure 12B).
Fig.
12A. A second action
potential is only produced when sufficient time has passed for the membrane
voltage to return to nearly the resting potential. Pulse #1 t1 = 1.00 ms and Pulse#2 t2 = 5.00 ms.
Note: in this instance the second spike has a smaller
amplitude than the initial spike.
not sure if the calculation of refractory times are correct!
Fig.
12B. A second action potential is
not produced if there is insufficient time for the neuron to recover. Pulse
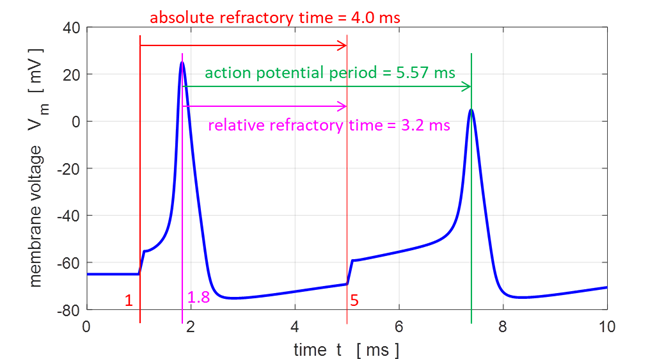
#1 t1 = 1.0 ms and Pulse#2 t2 = 4.9 ms. Refractoriness is the fundamental property of excitable medium not to respond
on stimuli, if the object stays in the specific refractory state. The refractory period is
a period of time during which a neuron is incapable of repeating another
action potential. It is the amount of time it takes for an excitable membrane
to be ready for a second stimulus once it returns to its resting state
following an excitation. The absolute refractory period
corresponds to depolarization and repolarization, whereas relative
refractory period corresponds to
hyperpolarization. The time interval between action potential peaks is 5.57 ms. So, if the neuron was stimulated by a series of
square pulses, the maximum frequency of the spikes is about 180 Hz. Refractoriness occurs, firstly due to the
hyperpolarizing spike after-potential which is lower than the resting
membrane potential. Hence, more time is needed to reach the
Fig.
12C. The first pulse occurs
at time 1.0 ms and the second pulse at 3.0 ms. In the short time interval between the pulses the
gating variables have not reached their rest state values. Hence, a large
proportion of the ion channels are open. Multiple current
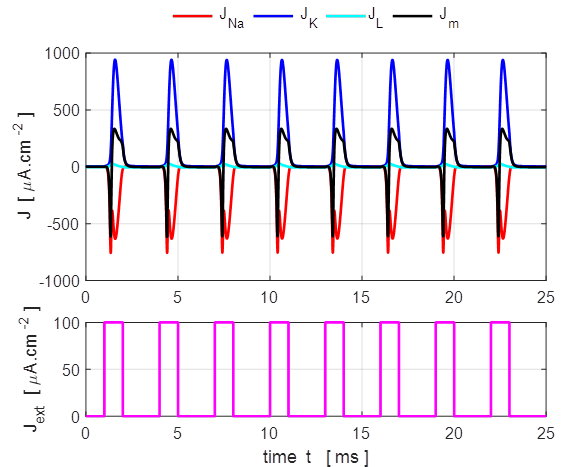
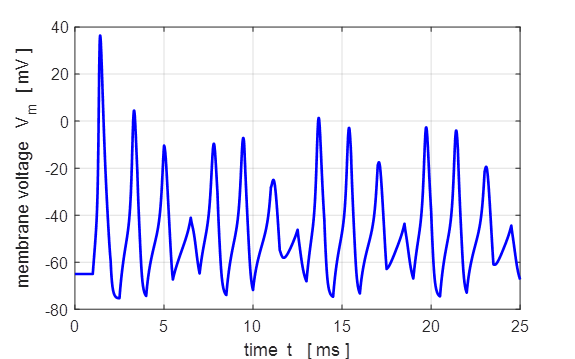
pulses flagJ = 4 You can study the response of the membrane potential
to a series of square pulses of uniform amplitude. Default input parameters case 4 % Series of current pulses % Amplitude of pulses [100e-6 A.cm-2] J0
= 100e-6; % Simulation time [2.5e-3 s] tMax = 25e-3; % Stimulus applied tSTART = 1e-3; % Pulse ON / OFF times tON = 1e-3; tOFF = 2e-3; % Number of grid points [8001] num = 8001;
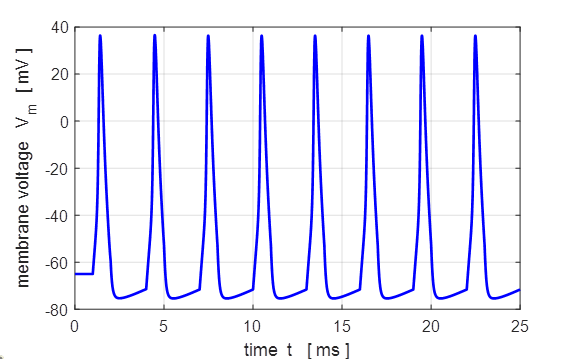
Fig.
13A. The
stimulus is applied at time t = 0.1 ms.
The pulse amplitude is 100
Fig.
13B. If the pulse rate is too rapid, then not all action potentials are
generated and a regular firing pattern is not established. The simulation
parameters for figure 13B are the same as figure 13A except the OFF time of
the pulse is reduced to 0.5 ms. Step current
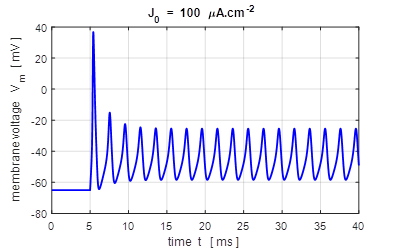
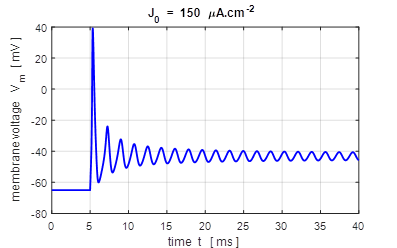
input flagJ = 2 We can model the response of the membrane potential
to a step input. Figure 14 shows the membrane potential for a series of step
functions with increasing amplitude. Default input parameters case 2 % Constant current injection step function % Amplitude of step function [100e-6 A.cm-2]
J0 = 100e-6; % Simulation time [40e-3 s]
tMax = 40e-3; % Time stimulus applied [5e-3 s]
tStart = 5e-3; % Number of grid points [8001]
num = 8001;
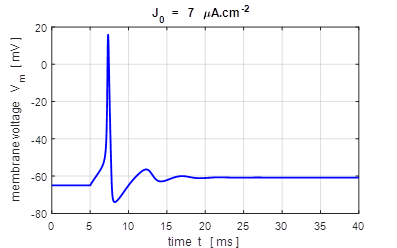
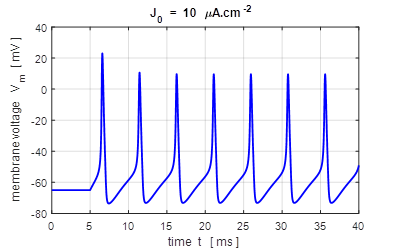
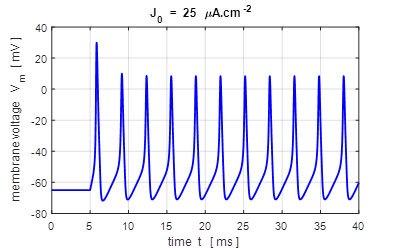
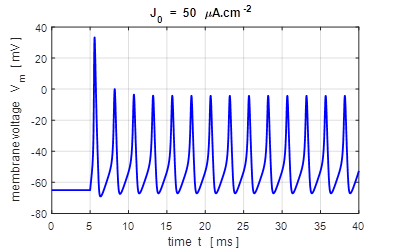
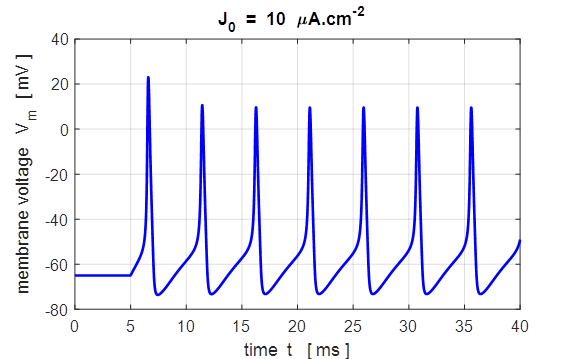
Fig.
14. A constant current
injection is used to stimulate the neuron. The stimuli are switched on at
time t
= 5.0 ms. If the size of the step is less than 7
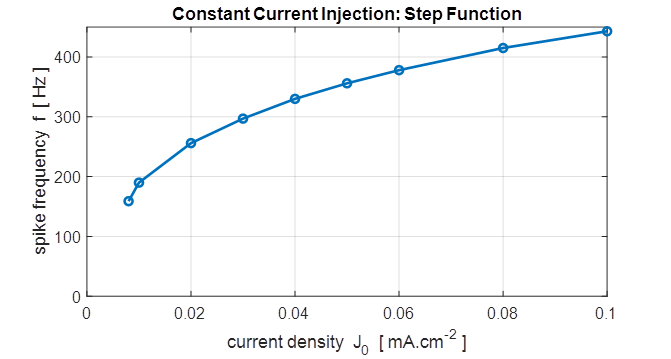
Fig.15A. The frequency f of
the repetitive firing was determined for each value of J0. This frequency is known as the firing rate. The curve is known as the gain function. This was done by using the
Matlab command ginput to measure the period of the
repetitive firing of the neuron ( inter-spike interval T
)
in the Figure Window for the variation in membrane potential as a function of
time where f = 1/T . bp_neuron_01bb.m
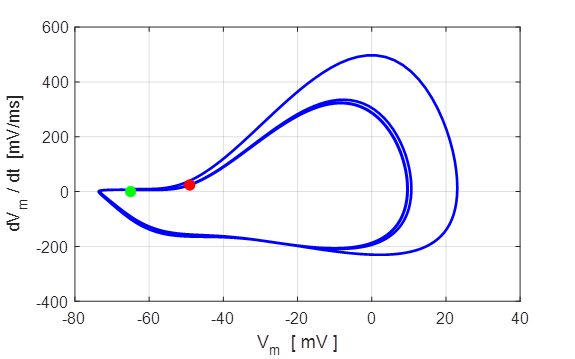
Fig.
15B. The regular spiking of the
neuron is clearly shown in the phase portrait plot. The stimulus input
current is called a heavy side step function. Pulse stimulus
with noise flagJ
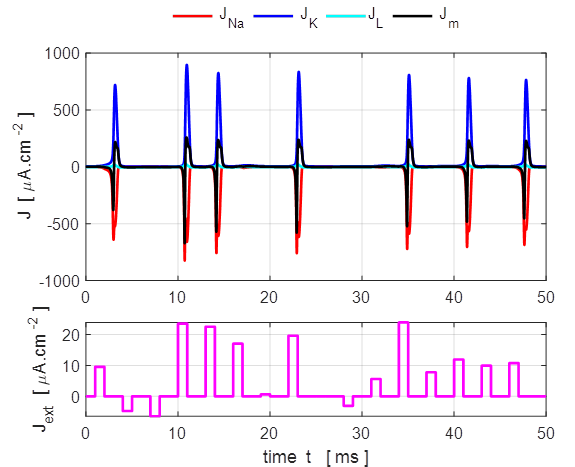
= 6 Default input parameters case 6 % Noise % Amplitude of step function [7e-6 A.cm-2] J0
= 7e-6; % Simulation time [2.5e-3 s] tMax = 50e-3; % Stimulus applied tSTART = 1e-3; % Pulse ON / OFF times tON = 1e-3; tOFF = 2e-3; % Number of grid points [8001] num = 8001;
The stimulus corresponds to a series of square
pulses whose amplitude is less than the threshold value. Then for each pulse,
noise is added using the rand
function. A neuron receives signals from thousands of other neurons creating
a noisy input resulting in small random fluctuations of the membrane
potential around its resting value. The noisy input can lead to a stimulus
strong enough to create a depolarization of the membrane producing a spike or
a short spike train.
Fig. 16A. Pulse current
injection stimulation with noise.
Fig.
16B. The stimulus is a
series of injected current pulses with an amplitude
less than threshold. Random noise is added to each pulse. A spike train is
produced where the spikes are produced spasmodically. Square pulse: J0 =
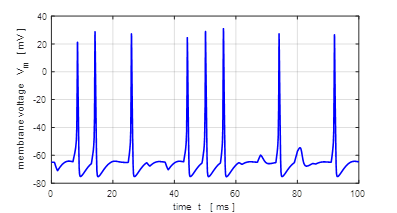
0.005 mA.cm-2, tON = 1.0 ms, tOFF = 5.0 ms. Sinusoidal external stimulus flagJ = 5 Default input parameters
case 5 % Sinusoidal current stimulus % Amplitude of step function [10e-6 A.cm-2] J0
= 10e-6; % Simulation time [50e-3 s] tMax = 50e-3; % Frequency of stimulus [200 Hz] pf = 200; % Number of grid points [1001] num = 1001; The excitation of nerve
cells by sinusoidal alternating current waveforms is very dependent upon the
frequency of the stimulus because of the necessity to transfer a specific
amount of charge to produce the excitation.
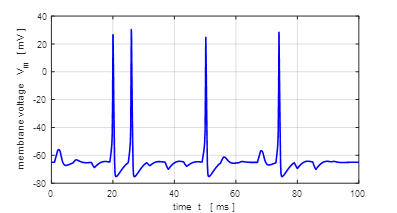
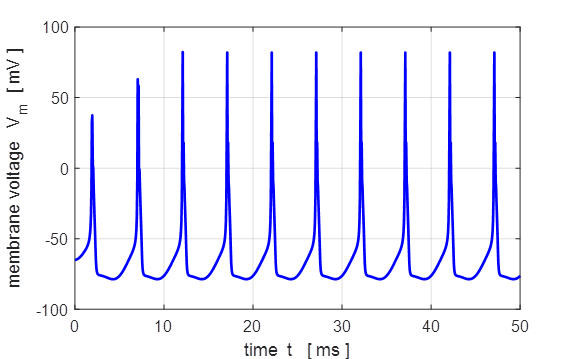
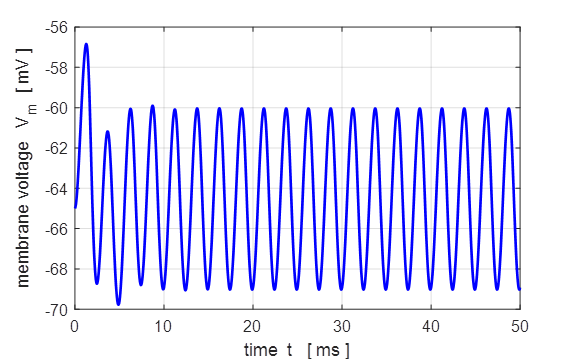
Fig. 17
. A sinusoidal external
current stimulus (amplitude 10.0
Fig.
18. A sinusoidal external
current stimulus (amplitude 10.0 The equations of Hodgkin
and Huxley provide a good description of the electrophysiological properties
of the giant axon of the squid. These equations capture the essence of spike
generation by sodium and potassium ion channels. The basic mechanism of
generating action potentials is a short in influx of sodium ions that is
followed by an efflux of potassium ions. Cortical neurons in vertebrates,
however, exhibit a much richer repertoire of electrophysiological properties
than the squid axon studied by Hodgkin and Huxley. These properties are
mostly due to a larger variety of different ion channels. |