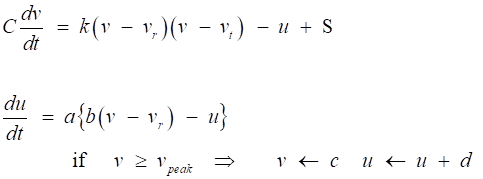|
|
IZHIKEVICH
QUADRATIC MODEL FOR SPIKING NEURONS |
|||||
|
ns_Izh_006.m
Computation of the firing patterns of a single neuron using the Izhikevich Quadratic Model |
||||||
|
This article and the Scripts are based upon the papers and book by E.
M. Izhikevich Which Model to Use for Cortical
Spiking Neurons? IEEE
Transactions on Neural Networks. Vol. 15. No. 5. September 2004. Simple Models of Neural Networks IEEE Transactions on Neural
Networks. Vol. 14. No. 6.
November 2003. Hydrid Spiking Models Phil. Trans. R. Soc. A (2010) 368,
5061–5070 Dynamical
Systems in Neuroscience: The Geometry of
Excitability and Bursting. The MIT Press. |
||||||
|
The
model of a neuron described in the book, Dynamical
Systems in Neuroscience: The Geometry of Excitability and Bursting by Izhikevich is not
based upon biophysical parameters but is a simple model that faithfully
reproduces all the neurocomputational dynamical
features of the neuron. The model is a two-dimensional systems having a fast
voltage variable v and
a slower “recovery” variable u, which may describe activation of the K+
current or inactivation of the Na+ current or their combination.
The simple model to reproduce spiking and bursting behavior of many known
types of neurons is described by the pair of differential equations
(1)
|
||||||
|
Model parameters and dimensions: t
time [ms] C membrane
capacitance [pF
= pA.ms.mV-1] v
membrane
potential [mV]
vr resting
membrane potential
[mV] vt instantaneous
threshold potential
[mV] k
constant
(“1/R”)
[pA.mV-1 (10-9 Ω-1)] u
recovery
variable [pA] S
stimulus
(synaptic: excitatory or inhibitory, external, noise) [pA]
a
recovery time constant [ms-1] b
constant (“1/R”) [pA.mV-1 (10-9 Ω-1) ] c
potential
reset value
[mV] d outward
minus inward currents activated during the spike and affecting the
after-spike behavior [pA] vpeak spike
cutoff value [mV] |
||||||
|
The sum of all slow currents that modulate
the spike generation mechanism is combined in the phenomenological recovery
variable u
with outward currents taken
with the plus sign. The sign of b determines
whether u
is an amplifying (b < 0) or a resonant (b > 0) variable. In the latter case (b > 0) the neuron sags in response to
hyperpolarized pulses of current, peaks in response to depolarized
subthreshold pulses, and produces rebound (postinhibitory)
responses. The parameters c and
d
do not affect steady-state
subthreshold behavior. Instead, they take into account the action of
high-threshold voltage-gated currents activated during the spike, and affect
only the after-spike transient behavior. One difficulty in applying the Izhikevich model is the determination of the numerical
values of the parameters to be used in stimulations of the time evolution of
the membrane potential for different stimuli. The following simulations show
how the model can be applied to three types of neurons. |
||||||
|
1. Modelling Regular Spiking (RS)
Neurons Regular spiking
neurons are the major class of excitatory neurons in the neocortex (part of
the cortex of the brain made up of six layers, labelled from the outermost
inwards, I to VI). In humans, the neocortex is involved in functions such as
sensory perception, generation of motor commands, spatial reasoning and
language. RS neurons have a transient K+ current IA whose
slow inactivation delays the onset of the first spike and increases the interspike period, and a persistent K+ current
IM
which is believed to be responsible for the spike frequency
adaptation. Regular spiking
neurons fire tonic spikes with adapting (decreasing) frequency in response to
injected pulses of DC current. Most of them have Class 1 excitability in the
sense that the interspike frequency vanishes when
the amplitude of the injected current decreases. These neurons are spiny
stellate cells in layer 4 and pyramidal cells in layers 2, 3, 5, and 6. The
simulated voltage responses of the model agree quantitatively with the in
vitro recordings of the layer 5 pyramidal neuron. Model
parameters for RS neurons in the neocortex: Membrane
resting membrane vr = −60
mV Instantaneous
threshold potential vt = −40
mV (instantaneous
depolarizations above −40 mV cause the neuron to fire) The
minimal amplitude of injected current of infinite duration needed to fire a
neuron is called the rheobase The parameters k and b can be found when one knows the neuron’s rheobase and input resistance rheobase =
50 pA
Input resistance R = 80 MΩ Þ k = 0.7 and
b
= −2. how ? Membrane
capacitance C = 100
pF Þ membrane time constant t = R C = 8 ms When
b <
0, the depolarizations of v
decrease u as
if the major slow current is the inactivating K+ current IA The
inactivation time constant of IA
is around 30 ms in
the subthreshold voltage range Þ a ≈
1/30 ≈ 0.03 The membrane
potential of a typical RS neuron reaches the peak value vpeak =
+35 mV during a spike and then
repolarizes to c = −50
mV or below, depending on the firing frequency The parameter d describes
the total amount of outward minus inward currents activated during the spike
and affecting the after-spike
behavior
d =
100 gives a reasonable f-I
(or f-S)
relationship in the low-frequency range. All the
figures 1… were created using the Matlab mscript ns_Izh_006.m. with
the parameters: C = 100; vr = -60; vt = -40; k =
0.7; % parameters used for RS a = 0.03; b =
-2; c = -50; d = 100;
% neocortical pyramidal neurons vPeak = 35;
% spike cutoff [mV] dt = 0.5;
% time step [ms] |
||||||
|
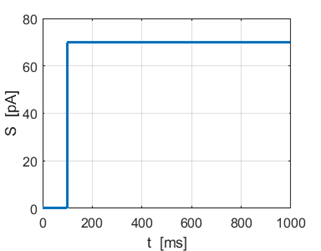
Fig. 1A. Input stimulus Sstep = 70 pA. |
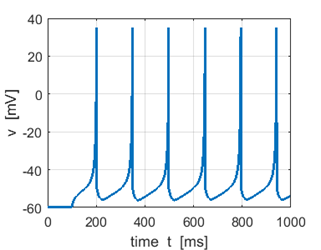
Fig. 1B. Membrane potential. |
|||||
|
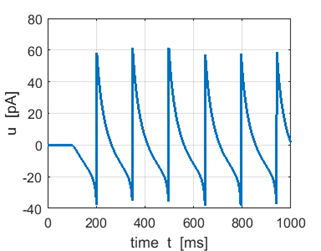
Fig.1C. Recovery variable. |
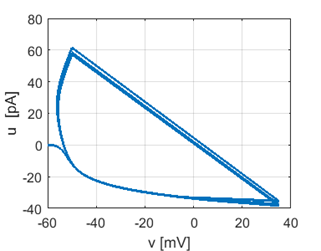
Fig.
1D. Phase plot. |
|||||
|
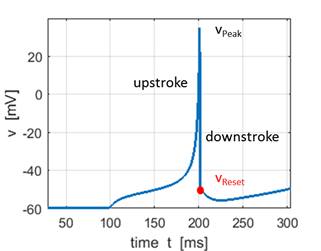
Fig.1E. Expanded view of a spike. The
shape of the action potential is very similar to the recording of actual
neocortical pyramidal neurons but with two discrepancies: recording has a
sharper spike upstroke and a smoother spike downstroke. The simple model generates
the upstroke of the spike due to the intrinsic (regenerative) properties of
the voltage equation. The voltage reset occurs not at the threshold, but at
the peak, of the spike. The firing threshold in the simple model is not a
parameter, but a property of the bifurcation mechanism of excitability.
Depending on the bifurcation of equilibrium, the model may not even have a
well-defined threshold, a situation similar to many conductance-based models. Pyramidal
neurons (pyramidal cells) are a type of neuron found in areas of the brain
including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal
neurons are the primary excitation units of the mammalian prefrontal cortex
and the corticospinal tract. Key features: triangular shaped soma (after which
the neuron is named), a single axon, a large apical dendrite, multiple basal
dendrites, and the presence of dendritic spines. |
Resting membrane potential vr = −60 mV Instantaneous threshold potential vt = −40 mV Instantaneous depolarizations above −40 mV cause the neuron to fire The slow afterhyperpolarization (AHP)
following the reset that is due to the
dynamics of the recovery variable u. |
|||||
|
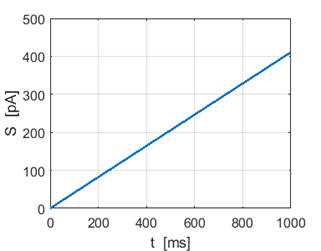
Fig.
1F. Ramp stimulus. |
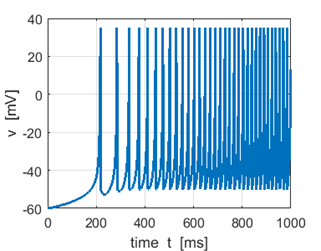
Fig.
1G. Membrane potential:
spike frequency increases as stimulus strength S
increases. |
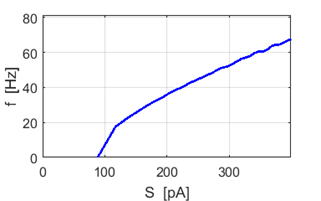
Fig.
1H. f-S curve showing how the spike frequency
increases with increasing stimulus strength. |
||||
|
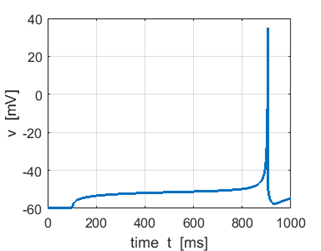
Fig. 1I.
Step input Sstep = 52 pA produces a
spike, but no spike is generated for Sstep = 51 pA. |
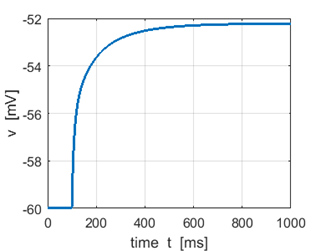
Fig.
1J. Step input Sstep = 51 pA. The minimal amplitude of the injected current of
infinite duration needed to fire a neuron is called the rheobase. |
|||||
|
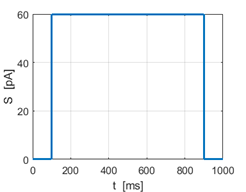
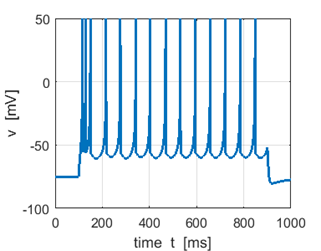
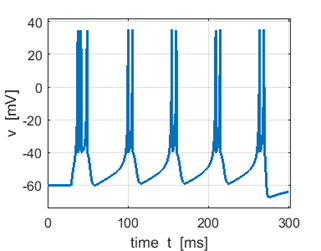
Fig. 1K. Step input
stimulus Sstep =
60 pA |
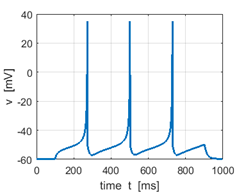
Sstep = 60 pA Mean firing rate f = 4.4 Hz |
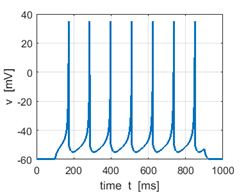
Sstep = 80 pA f = 8.9 Hz |
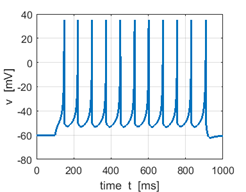
Sstep =
100 pA f =
13.1 Hz Increasing the input stimulus strength increases the firing rate |
|||
|
|
||||||
|
2. Modelling Intrinsically Bursting
(IB) Neurons |
||||||
|
Intrinsically bursting (IB)
neurons generate a burst of spikes at the beginning of a strong depolarizing
pulse of current, then switch to tonic spiking mode. They are excitatory
pyramidal neurons found in all cortical layers, but are most abundant in layer
5. Some IB
neurons respond to the injected pulses of DC current with a burst of
high-frequency spikes followed by low-frequency tonic spiking. Many IB
neurons burst even when the current is barely superthreshold
and not strong enough to elicit a sustained response. However, other IB
neurons give a bursting response only to strong current stimuli and weaker
stimulation elicits a regular spiking response. In comparison with typical RS
neurons, the regular spiking response of IB neurons have a lower firing frequency
and higher rheobase (threshold) current, and
exhibits shorter latency to the first spike and noticeable
afterdepolarizations. The initial high-frequency
spiking is caused by the excess of the inward current or the deficit of the
outward current needed to repolarize the membrane potential below the
threshold. As a result, many spikes are needed to build up outward current to
terminate the high-frequency burst. After the neuron recovers, it fires
low-frequency tonic spikes because there is a residual outward current (or
residual inactivation of inward current) that prevents the occurrence of
another burst. Many IB neurons can fire two or more bursts before they switch
into tonic spiking mode. Default model
parameters for IB neurons for a step input as in figure 1K: C =
150 vr = -75 vt
= -45 k =
1.2 a =
0.01 b = 5 c = -56 d = 130 vPeak
= 50 Rheobase = 350 pA input resistance ~
30 MΩ |
||||||
|
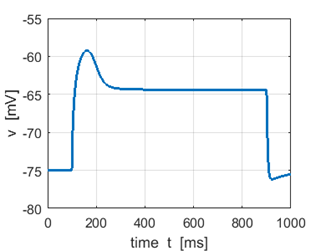
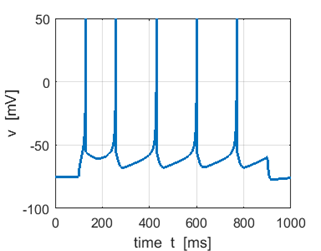
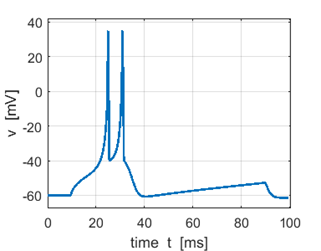
Fig. 2A. Sstep = 300 pA. The input
current is less than the rheobase, therefore, the
neuron does not fire. |
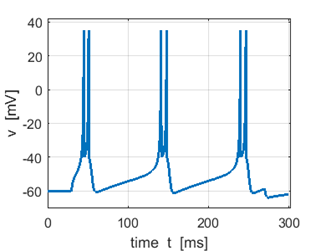
Fig. 2B. Sstep = 400 pA Low frequency tonic
spiking with adaptation since the input stimulus is only slightly greater
than the rheobase value. |
|||||
|
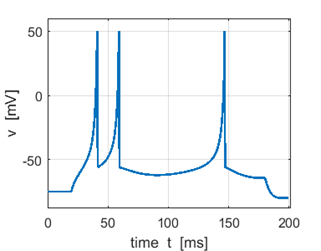
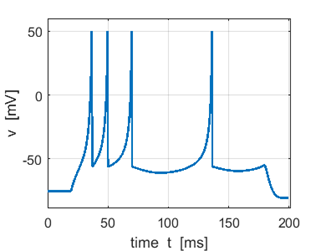
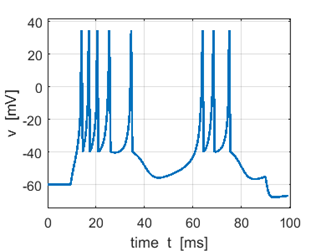
Fig. 2C. Sstep = 500 pA. The first
spike is a doublet. |
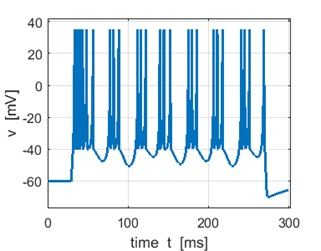
Fig. 2D. Sstep = 600 pA. the first
spike is a triplet. |
|||||
|
|
||||||
|
3. Modelling Chattering (CH)
Neurons |
||||||
|
Chattering (CH) or fast
rhythmic bursting (FRB) neurons generate high-frequency repetitive bursts in response
to injected depolarizing currents. The magnitude of the DC current determines
the interburst period, which could be as long as
100 ms or as short as 15 ms
and the number of spikes within each burst is typically from two to five. CH neurons are found in visual cortex of adult
cats, and morphologically they are spiny stellate or pyramidal neurons of
layers 2 - 4, mainly layer 3. Default model
parameters for IB neurons for a step input as in figure 1K: C =
50 vr = -60 vt
= -40 k =
1.5 a =
0.03 b = 1 c = -40 d = 150 vPeak
= 35 The results of the simulations
are shown in figures 3…. The plots are very similar to vivo recordings from cat primary visual cortex. |
||||||
|
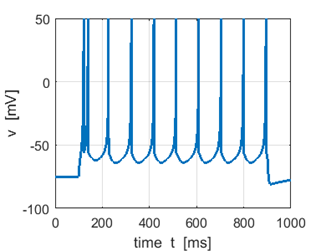
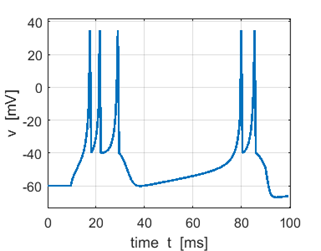
Fig. 3A. Sstep = 200 pA. Doublet spikes. |
Fig. 3B. Sstep = 300 pA. Triplet first spike. |
|||||
|
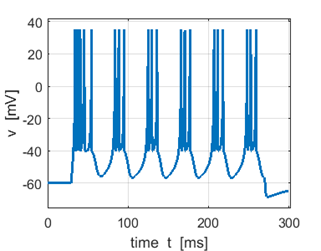
Fig. 3A. Sstep = 500 pA. Multiple spikes. |
Fig. 3A. Sstep = 600 pA. Multiple spikes. |
|||||
|
The
model described by equation 1 can quantitatively reproduces subthreshold,
spiking, and bursting activity of all known types of cortical and thalamic
neurons in response to pulses of DC current. The simple model makes testable
hypotheses on the dynamic mechanisms of excitability in these neurons and the
model is especially suitable for simulations of large-scale models of the
brain. |
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
|
|||||
|
|
||||||
|
|
||||||