|
COMPUTATIONAL NEUROSCIENCE A BRIEF INTRODUCTION TO NEURAL SYSTEMS Ian Cooper Any comments, suggestions or corrections, please email me at |
|
DOWNLOAD DIRECTORY FOR SCRIPTS bp_neuron_01.m
ns_Izh002.m
ns_Gon001.m |
|
NEURAL SYSTEMS Our body functions are controlled by
electrical and chemical systems. We can measure many of these electrical
signals to obtain useful information about the functioning of our bodies: EMG electromyogram (muscle function) ECG electrocardiogram (heart
function) EEG electroencephalogram (brain
functions) ERG electroretinogram
(eye functions) The nervous system is made of two parts: ·
The
central nervous system that controls voluntary functions. The central nervous
system consists of the brain, spinal cord and the peripheral nerves. Neurons
transfer information to the spinal cord and brain from sensors sensitive to
sound, light, smell, temperature, feel, etc. In response, signals are sent
from the brain through the spinal cord to activate muscles. ·
The
autonomous nervous system controls involuntary functions such as the inner
organs, heart and intestines. This system cannot be controlled voluntarily. Neurons or nerve cells are the elementary processing units in the
brain and central nervous system. The neurons form an intricate network of
connections. The human nervous system consists of about 1011
interconnected neurons. There are about 104 cell bodies of
cortical neurons and several kilometres of
connections within a volume element of 1 mL. This
complex network of neurons receives processes and transmits information from
one part of the body to another. When a neuron receives an appropriate stimulus,
it produces electrical pulses called action potentials that are
propagated along its cable-like structure. When a pulse reaches the end of
the nerve cell, other neurons or muscle cells may be activated. There are
three types of neurons: sensory neurons (receive stimuli from sensory
organs), interneurons (transfer information from one neuron to another), and motoneurons (transfer information about the control of
muscle cells). The sending neuron is referred to as the presynaptic cell and the receiving neuron as the postsynaptic cell. In the vertebrate cortex, a single
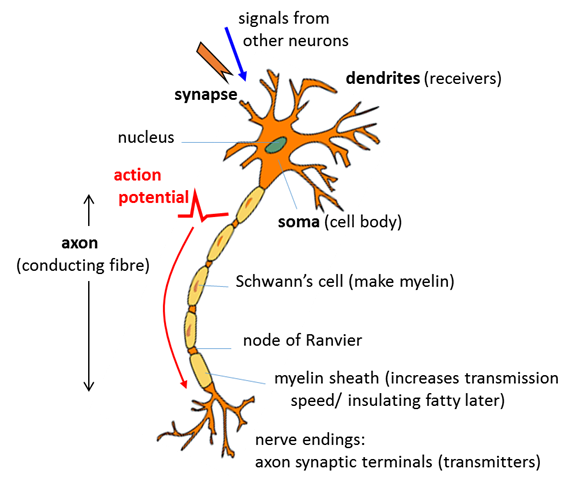
neuron can connect to more than 104 postsynaptic neurons. A typical neuron consists of three functionally distinct parts (figure
1): · Dendrites: input part - collects
signals from other neurons and transmits them to the soma. · Soma: processing part –
if the total non-linear input signal from the dendrites is greater than some
threshold, an output signal is generated. · Axon: output part – electric signals propagated away from the soma
to other neurons across junctions known as synapses. Some neurons are extremely long, for
example, the axon connecting our toes with the spine can be more than 1 m
long. Human axons are very thin with diameters about 20
Fig. 1. Sketch
showing the main parts of a neuron. Body fluids are good electrical conductors because salts and other
molecules dissociate into positive and negative ions. The inside of an axon
is filled with an ionic fluid that is separated from the surrounding body
fluid by a thin membrane that is from about 5 nm to 10 nm thick. The
ionic solutes in the extracellular fluid are mainly Na+ and Cl- ions. In the intracellular fluid, the
positive ions are mainly K+ and the negative ions are mainly large
negatively charged organic ions.
Hence, there is a large concentration of Na+ ions outside
the axon and a large concentration of K+ ions inside the axon. The
concentration of the different ion species does not equalize by diffusion
because of the special properties of the cell membrane. In the resting state
when the axon is non- conducting, the axon membrane is highly permeable to K+
ions, slightly permeable to Na+ ions and impermeable to large
negative organic ions. More K+ ions leak out of the cell than Na+
ions that leak into the cell. This leaves the inside of the cell more
negative than the outside. A potential difference therefore exists across the
cell membrane because of the difference in the concentration of ions in the
extracellular and intracellular fluids. This potential difference is called
the membrane potential vm(t). The outside of the cell
is taken as the reference potential 0 V. The resting membrane potential has a
strong negative polarization and is constant at about -65 mV. This negative
membrane potential restricts the further diffusion of the K+ to
the outside of the cell, so equilibrium is established where the electrical
forces balances the chemical forces. The mechanism for the generation of an electrical signal by a neuron
is conceptually simple. When a neuron receives a sufficient stimulus from
another neuron, the permeability of the cell membrane changes. As a result of
the changes in membrane permeability, the sodium ions first rush into the
cell while the potassium ions flow out of it. The movement of the ions across
the membrane constitutes an electric current signal which propagates along
the axon to its terminations. These membrane currents depolarize the cell so
that the interior of the cell becomes positive and a neuronal voltage signals
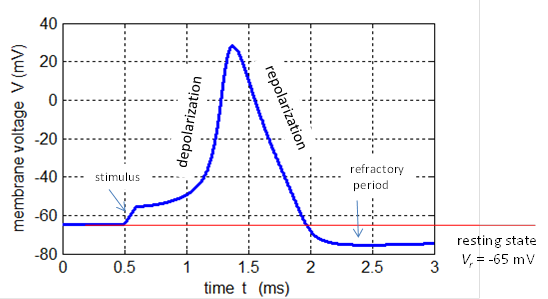
is generated. These short voltage pulses are called spikes or action potentials and have a
duration of less than a few milliseconds and have a peak about +20 mV (figure
2). The action potential propagates along an axon without a change in shape.
Fig. 2. Action potential produced by an external current pulse (Jext
= 1.0x10-4 A.cm-2 and duration 0.10 ms)
at a temperature of 18.5 oC. The plot was created using the
Hodgkin-Huxley Model with the Script bp_neuron_01.m. A sequence of spikes from a neuron is called a spike train and the timing between spikes maybe regular of irregular.
A single spike does not carry useful information. It’s the pattern or
timing of the spikes that is important in the neural dynamics of the brain.
The minimal time interval between two spikes of a single neuron is called the
absolute refractory period. It is not possible for a
neuron to generate a second spike in this period, even with a strong input.
In a short-time interval called the relative
refractoriness phase after the absolute refractory, it is difficult, but not
impossible to excite another action potential.
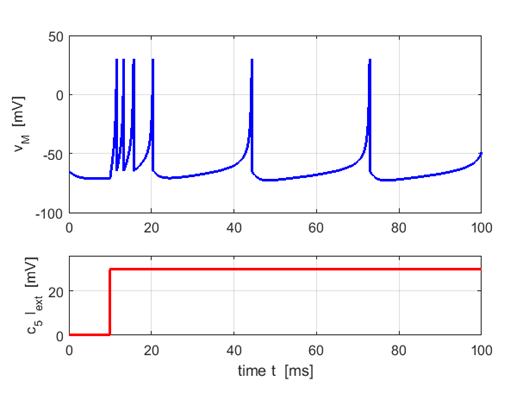
Fig. 3.
An example of a spike train known as tonic spiking with spike
frequency adaption. Plot created using the Script ns_Izh002.m. The synapse
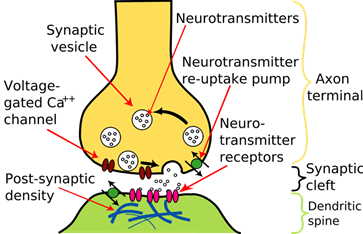
is the junction between axons of presynaptic neurons and the dendrites of postsynaptic
neurons. There are two types of synapses in which neurons are coupled
together, chemical and electrical. Chemical synapses are the most common synapses in the vertebrate
brain. The chemical synapse is the very small gap between a terminal axon of
the presynaptic neuron and the dendrites of the postsynaptic neuron. This gap
is called the synaptic cleft.
The action potential arriving at the termination of the axon of the
presynaptic neuron triggers a complex chain of complex bio-chemical events: ·
Release of a
neurotransmitter into the synaptic cleft ·
Detection of the
neurotransmitter by specialized receptors in the postsynaptic neuron ·
Ion channels open up
in the postsynaptic membrane leading to an influx of ions from the
extracellular fluid into the cell causing a change in membrane potential
generating the postsynaptic potential. The release of neurotransmitters may be excitatory and increase the
membrane potential which may lead to the generation of a spike or if the
change in membrane potential is negative (no spike can be produced), the
synapse is inhibitory. The cell membrane is depolarized by an input at an excitatory synapse which
reduces the negative polarization. An input that increases the negative
polarization of the membrane even further is called hyperpolarizing.
Fig. 4. Schematic
diagram of the synapse of a neuron We can consider the role of the
synapses in the time evolution of the membrane potential (1) where the right hand side of equation 1
defines the postsynaptic potential (PSP). If
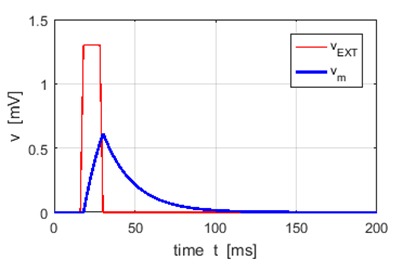
Fig. 5. A neuron receives an external
excitatory voltage input stimulus from a set of presynaptic neurons. The
membrane potential increases to a voltage less than the threshold voltage vTH = 1.0 mV and then falls to its
resting potential The neuron m receives a PSP each time the neuron n fires. Also, the neuron m will receive PSPs
from not only neuron n but also
from many neurons. We assume the total PSP input to
neuron m is the sum of the PSPs produced from the repeated firing of many
neurons. This linearity however
breaks down if too many input spikes arrive during a short interval. Single EPSPs have ~ 1 mV amplitudes and the threshold
value for spike initiation is ~ 25 mV above the resting potential. Therefore,
about 20-50 presynaptic spikes within a short time window are necessary for
the firing of an action potential (short duration voltage pulse with an
amplitude ~ +100 mV). After the firing of the action potential, the membrane
potential undergoes a phase of hyperpolarization below the resting value
called the spike-after-potential (figure 2). The action potential once
generated propagates along the axon of the neuron to the synapses of other
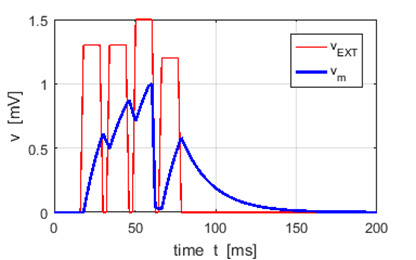
neurons. Figure 6 shows a schematic diagram of the series of input stimuli
that triggers an action potential. Spiking neuron models such as
integrate-and-fire model (LIF) model are referred
to as Spike Response Models (SRM). Spike Response
Models provide are a useful conceptual framework for the analysis of neuronal
dynamics and neuronal coding.
Fig. 6. A schematic diagram for the
triggering of an action potential due to the summation of a series of input
stimuli from a set of presynaptic neurons. The plot was produced with the Script ns_Gong001.m using the leaky
integrate-and-fire model (LIF). The LIF model only resets the membrane potential to the
resting value when the membrane potential reaches the threshold potential. |