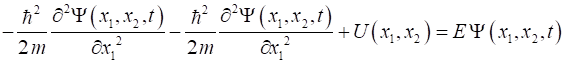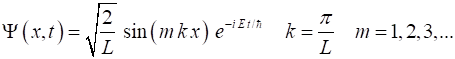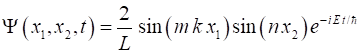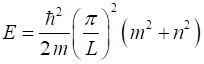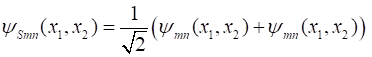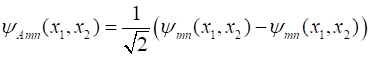|
TWO PARTICLES IN AN INFINITE POTENTIAL
WELL Symmetric and Antisymmetric
States Bosons and Fermions Ian Cooper Any
comments, suggestions or corrections, please email me at matlabvisualphysics@gmail.com |
|
MATLAB DOWNLOAD
DIRECTORY FOR SCRIPTS qmTwo.m Calculation of the
probability density function of finding two particles confined within an
infinite potential well. Within the Script you can change the quantum numbers
m and n. The Script can be modified to a Live Editor Script to better observe
the changes in the probability density as the values of m and n are changed. |
|
Consider two
particles confined in an infinite potential well (particle in a box) (1)
Assume that the
particles do not interact with each other. Then by analogy with our
construction of the Schrodinger equation for a single particle, the
Schrodinger equation for our two particles becomes (2) The solution can be
written as a product of the wavefunctions for each particle
(3)
The solution of the
Schrodinger equation for a particle in a box is
(4) Therefore, a
solution Schrodinger equation for our two particles in a box on can be
expressed as (5) The energy E of the state for quantum numbers (6) How do we interpret the wavefunction? The probability of
finding particle 1 in a small line length (7) For the state (2,
3), in a very small interval
Now, we can
calculate the probability density of finding particle 2 at position
The problem is this, we have assumed that the particles are identical.
We can’t tell which is which, and nobody else can either. The indistinguishability
of elementary particles is not like that of apparently identical macroscopic
objects, where one could always place some tiny mark. There is no way to mark an
electron. This means that the
best we can do is to talk about the probability of finding one electron at
position (8) From the above
example
Therefore, the
wavefunction given by equation 5 is not a physically meaningful wave function
for two identical particles in a one-dimensional box. However, this
problem can be easily fixed by having different linear combinations of
wavefunction given by equation 5 that form symmetric or antisymmetric wavefunctions that satisfy the Schrodinger equation,
have the same energy E and satisfy
the requirements when the two particles are swapped around. Symmetric Function (9A) Antisymmetric Function (9B) Both symmetric and antisymmetric wavefunctions arise in nature in describing
identical particles. All elementary particles are either fermions, which have antisymmetric multiparticle wavefunctions, or bosons which have symmetric
wavefunctions. Electrons,
protons and neutrons are fermions; photons, alpha particles and helium atoms
are bosons. It is important to
realize that this requirement of symmetry of the probability distribution,
arising from the true indistinguishability of the
particles, has a large effect on the probability distribution, and,
furthermore, the effect is very different for fermions and bosons. This can be shown by plotting the
probability distribution functions for symmetric and antisymmetric
states and then comparing them. The Script qmTwo.m is used to plot the probability density functions for
our two particles confined in the box so that you can compare the plots for
symmetric and antisymmetric states for different
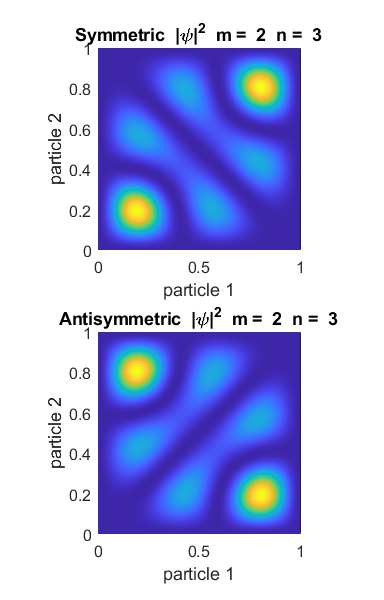
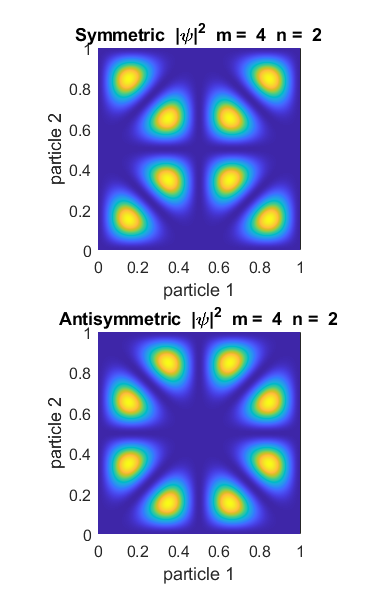
values of m and n. Figure 1 shows the example for m = 2 and n = 3.
Fig. 1. Symmetric and antisymmetric states for m = 2 and n = 3. The probability
density functions are very different. For the symmetric state, the highest
probability is that the two particles are close together (x1 = x2 = 0.2 and x1 = x2 = 0.8). Such
particles are called bosons. However, for the antisymmetric case, the particles are most likely to be
found far from each other. In fact, there is zero probability that they will
be found at the same spot, because The plots below show
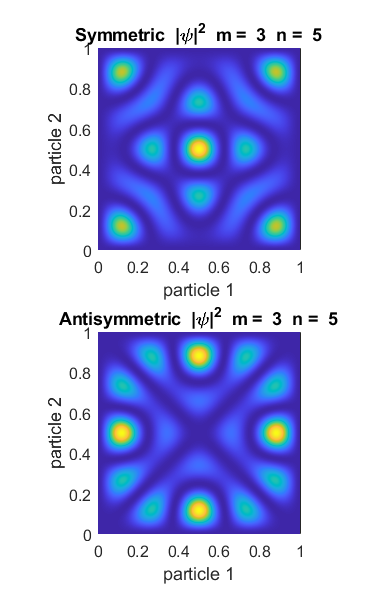
states with different quantum numbers. For each symmetric state it is most
likely that the particles will be close together and far apart for antisymmetric states.
|