|
3.2
THERMODYNAMICS P32 002 A A
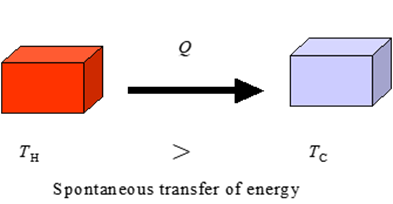
small metal nail and a large iron bolt are placed into a hot oven and removed
many hours later. What can you conclude about the temperature and the internal
energy of the nail and bolt? The
nail and bolt are dropped into two identical containers of water which are at
the same temperature. Comment on the rise in temperature of the two
containers of water. B What
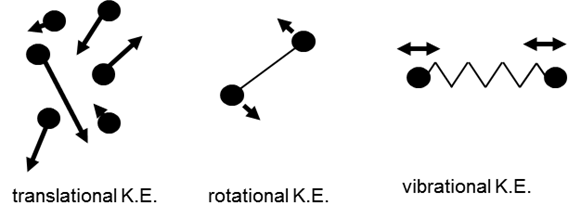
is meant by the terms translational kinetic energy, vibrational kinetic
energy and rotational kinetic energy? How do these terms relate to the
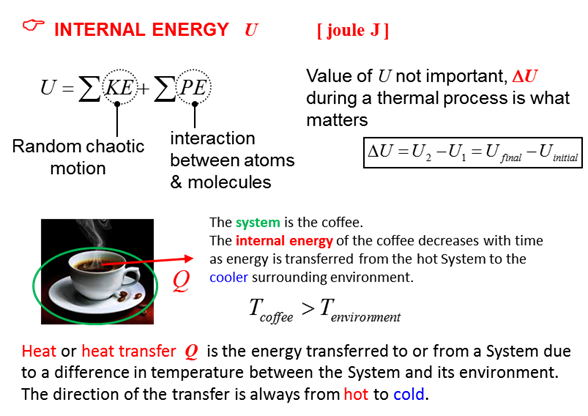
concepts of temperature, heat, thermal equilibrium and internal energy? C What
is meant by the statement, a thermometer measures its own temperature? View solution below only after you have completed the answering the question. |
|
Solution A The nail and
bolt will come to an equilbrium tmeperature in the oven, so they will have the same
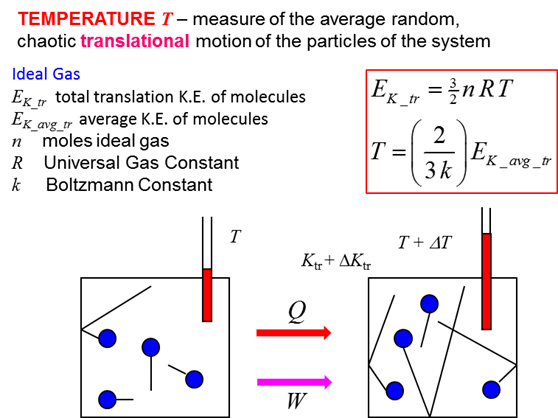
temperature when removed. The average translational kinetic of the molecules
in the nail is equal to the average translational kinetic energy of the
molecules in the bolt. The mass of the
bolt is greater than the nail (the bolt has many more molecules than the
nail). Hence, the bolt has more internal energy than the nail. The internal
energy is the total random energy due to the molecular motion of the
molecules of the object. When they are dropped in the containers of water,
more energy can be transferred from the bolt than the nail because there are
more molecules in the bolt for energy to be transferred to the water. Hence,
the water container with the bolt will reach a greater temperature. B Molecules
always have some random or chaotic motion. Therefore, a System
has a kinetic energy due to this random and chaotic motion. The kinetic energy
is classified as translational kinetic energy (movement of molecules from one place to
another), rotational
kinetic energy (rotation of
molecules about the XYZ axes) and vibrational kinetic energy (periodic vibrations of the molecules).
C A thermometer is an instrument that measures its own temperature. Many physical properties change in response to a change in temperature. For example, in a mercury thermometer, the mercury expands as its temperature increases. To measure a temperature accurately, then the thermometer must be in thermal equilibrium with its surroundings. Two physical systems are in thermal equilibrium if there is zero net flow of thermal energy between them when they are connected by a path permeable to energy transfer.
|
|
VISUAL PHYSICS ONLINE http://www.physics.usyd.edu.au/teach_res/hsp/sp/spHome.htm If you have any feedback, comments,
suggestions or corrections please email: Ian Cooper
School of Physics University of
Sydney ian.cooper@sydney.edu.au |