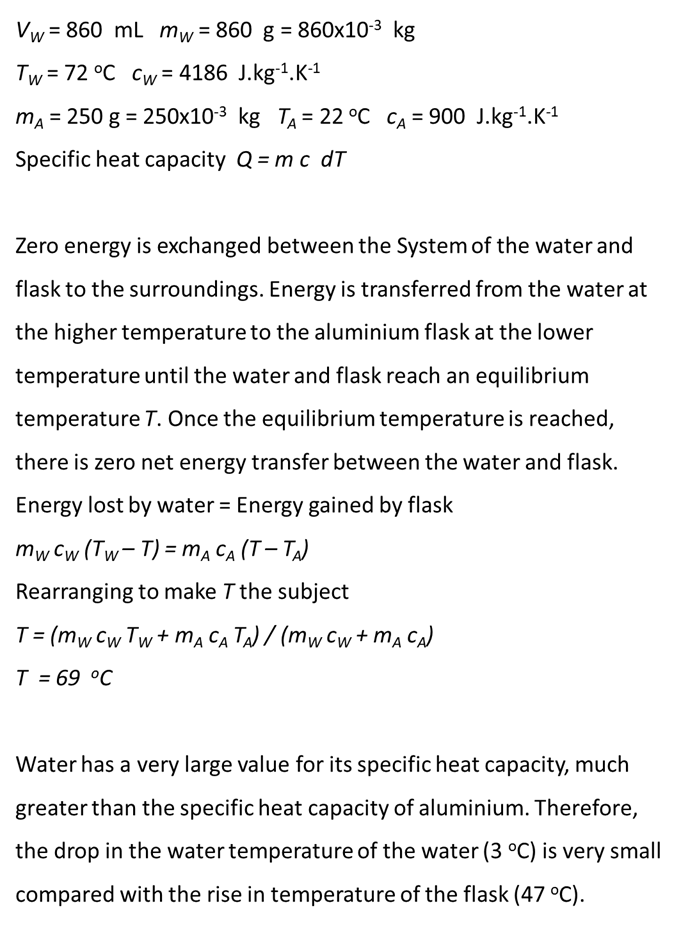
|
3.2
THERMODYNAMICS P32 004 Water
of volume 860 mL at 72.0 oC was poured
into an aluminium flask. The mass of the flask was 250 g and it had an
initial temperature of 22.0 oC. Final
the equilibrium temperature of the water and flask assuming zero heat
transfer with the surroundings. Explain
the heat transfers that occur. Comment
on the significance of the final equilibrium temperature making
reference to the concept of specific heat capacity. View solution below only after you have completed the answering the question. |
|
Solution
|
|
VISUAL PHYSICS ONLINE http://www.physics.usyd.edu.au/teach_res/hsp/sp/spHome.htm If you have any feedback, comments,
suggestions or corrections please email: Ian Cooper
School of Physics University
of Sydney ian.cooper@sydney.edu.au |