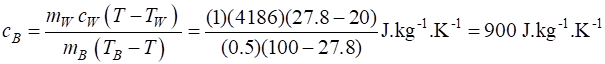|
3.2
THERMODYNAMICS P32 005 Outline
the steps you would need to perform to measure the specific heat of a metal
block. Compare your steps with the calculation need to solve the numerical
problem. A
0.500 kg metal block was place into a pot of boiling water. After many
minutes the metal block was removed from the pot and then dropped into an
insulated container holding 1.000 kg of water at 20.0 oC. The water and block in the insulator
container had a final temperature of 27.8 oC.
Find the specific heat of the metal. What metal is the block made of? Carefully state the assumptions you need to
make. cwater = 4186 J.kg-1.K-1 View solution below only after you have completed the answering the question. |
|
Solution Assumptions The metal block
was in equilbrium with the boiling water. Therefore, the intial temperature
of the block was TB =
100 oC. All the energy from the hot metal block was transferred to the water/block system to reach a steady equilibrium temperature T = 27.8 oC. Ignore any heating of the insulated container. Parameters Block mB = 0.5 kg cB = ? J. kg-1. K-1 initial temperature TB = 100 oC Water mW = 1.0 kg cW = 4186 J. kg-1 .K-1 initial temperature TW = 20.0 oC Final equilibrium temperature T = 27.8 oC Calculations Change in temperature caused by heat
transfer Q = m c
dT Energy is conserved – energy lost by block equals energy gained by block Heat lost by block QB
= mB cB
(TB - T) Heat gained by water QW = mW cW (T
– TW)
The block is made of aluminium.
|
|
VISUAL PHYSICS ONLINE http://www.physics.usyd.edu.au/teach_res/hsp/sp/spHome.htm If you have any feedback, comments,
suggestions or corrections please email: Ian Cooper
School of Physics University
of Sydney ian.cooper@sydney.edu.au |