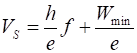|
VISUAL PHYSICS ONLINE 7.1 THE NATURE OF LIGHT ELECTROMAGNEITC WAVES P71 002 (A) What is the energy of a photon of wavelength 550 nm (joules and electron volts)? What is the frequency and what part of the electromagnetic spectrum for this photon? (B) A
student carried out an experiment during which light of different frequencies
was shone onto a metal surface to produce electrons.
The student measured the maximum kinetic energy of the emitted photoelectrons
as the frequency of light was altered. (B1) How did the student measure the
maximum kinetic energy? (B2) What
is the mathematical relationship between the maximum kinetic energy of the
photoelectrons and the frequency of the light incident on the metal surface?
Define each term in the equation and its SI unit. (B3) How
could the student best analyse the data to determine a value for
Planck’s constant? (C) Photoelectrons are emitted by a surface of a certain metal when the surface is illuminated by both violet light of wavelength 400 nm and green light of wavelength 550 nm but no photoelectrons are released from the surface by red light of wavelength 715 nm. (C1) Calculate the frequencies and the photon energies of the three light beams. (C2) Explain the differences in energies of the electrons released by the violet and green light. (C3) Explain why no electrons are released when illuminated by red light. View solution below only after you have completed the answering the question. |
|
Solution (A) E = ? eV = ? J f = ? Hz l = 550 nm = 550´10-9 m c = f l E = h f = h c / l f = c / l = 5.5´1014 Hz E = 3.6´10-19 J = 2.3 eV visible (B) Using a reverse voltage to reduce the photocurrent to zero à stopping voltage.
Plot Vs against f à slope = h/e à h (C) violet l1 = 400 nm = 400´10-9 m f1 = ? Hz E1 = ? J = ? eV green l2 = 400 nm = 400´10-9 m f2 = ? Hz E2 = ? J = ? eV red l3 = 715 nm = 715´10-9 m f3 = ? Hz E3 = ? J = ? eV
f1 = 7.5´1014 Hz E1 = 5.0´10-19 J = 3.1 eV f2 = 5.5´1014 Hz E2 = 3.6´10-19 J = 2.2 eV f3 = 4.2´1014 Hz E3 = 2.8´10-19 J = 1.7 eV Violet light photons have 0.9 eV more energy à released electrons will have 0.9 eV extra kinetic energy. The energy of the red photons is less than the work function of the surface à no electrons released from surface. |