|
VISUAL PHYSICS ONLINE 7.1 THE NATURE OF LIGHT
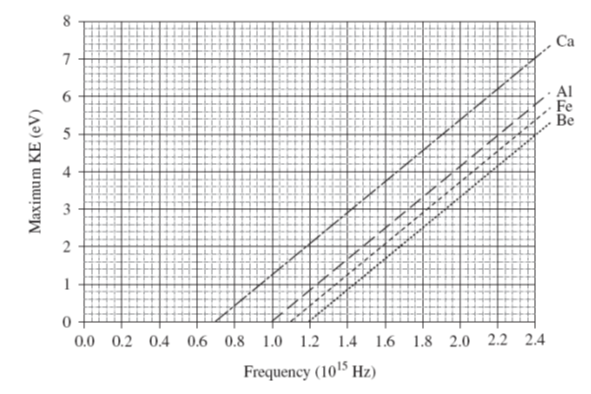
QUANTUM MODEL P71 012 When electromagnetic radiation shines on metals, photoelectrons
may be emitted. The maximum kinetic energy of emitted photoelectrons is
plotted against radiation frequency for four metals. Electromagnetic
radiation of wavelength 187 nm shines upon an unknown metal and the maximum
kinetic energy of the photoelectrons is found to be 2.5 eV. What is the
unknown metal?
View solution below only after you have completed the answering the question. |
|
Solution Incident radiation l = 187 nm = 187´10-9 m c = l f c = 3.00´108 m.s-1 f = c / l = 1.60´1015 Hz Max KE EKmax = 2.5 eV = (1.602´10-9)(2.5) J = 4.00´10-19 J Photoelectric Effect h f = EKmax
+ W
W = h fc Need to find threshold frequency fc f c = f – EKmax / h = 1.0´1015 Hz à Al
|