|
VISUAL PHYSICS ONLINE 8.1 FROM THE UNIVERSE TO THE ATOM
STRUCTURE OF THE ATOM P81 011 Our
understanding of matter has changed dramatically over time. Sketch a model of
the atom as proposed by each of the following three scientists: (A) Thompson (B) Rutherford
(C) Bohr View solution below only after you have completed answering the question. The solution is not in a form that you would answer in an examination. The answers are often in more detail to help improve your appreciation and understanding of the physics. |
|
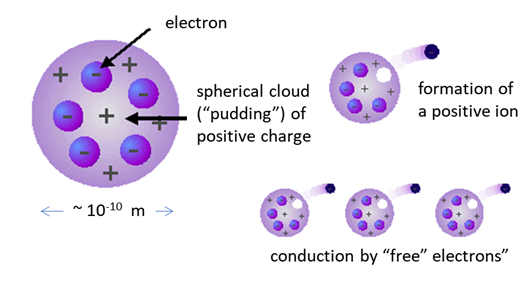
Solution (A) Plumb pudding model proposed by J J Thomson in 1904: electrons embedded in a positively charged sphere (like raisins in a pudding). J J Thomson is credited with the discovery of the electron.
(B) The Rutherford atomic model, (nuclear atom or planetary model) was the structure of atoms proposed in 1911 by the New Zealand-born physicist Ernest Rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
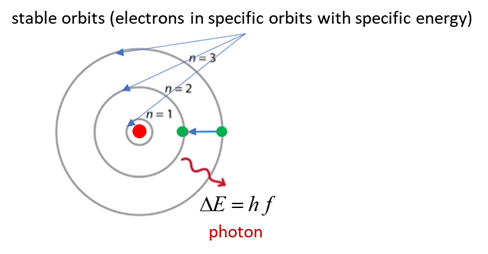
(C) In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus.
To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.
|