|
VISUAL PHYSICS ONLINE 8.1 FROM THE UNIVERSE TO THE ATOM
STRUCTURE OF THE ATOM P81 012 Nineteenth
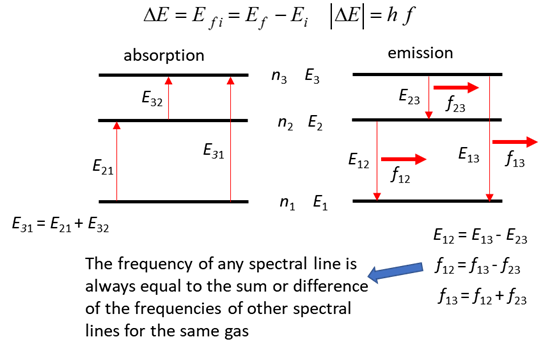
century physicists noted that for simple gases, the frequency of any spectral
line was always equal to the sum or differences of the frequencies of other
spectral lines for the same gas. Explain this fact using Bohr’s model
of the atom. View solution below only after you have completed answering the question. The solution is not in a form that you would answer in an examination. The answers are often in more detail to help improve your appreciation and understanding of the physics. |