|
VISUAL PHYSICS ONLINE 8.1 FROM THE UNIVERSE TO THE ATOM
STRUCTURE OF THE ATOM P81 013 Explain: According to classical
electromagnetic theory, an electron can not orbit the nucleus of an atom. Hence,
how did Bohr improve the Rutherford model of the atom? View solution below only after you have completed answering the question. The solution is not in a form that you would answer in an examination. The answers are often in more detail to help improve your appreciation and understanding of the physics. |
|
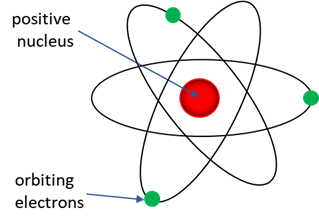
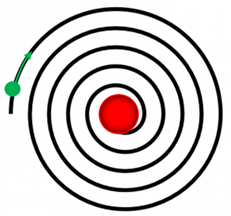
Solution According to classical physics, any charged object that is accelerating emits electromagnetic radiation and hence must loss energy. Therefore, an orbiting an electron (such as described in the Rutherford model) has a centripetal acceleration and must be emitting electromagnetic radiation as it losses energy and spirals into the positive nucleus. The Rutherford atomic model, (nuclear atom or planetary model) was the structure of atoms proposed in 1911 by the New Zealand-born physicist Ernest Rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
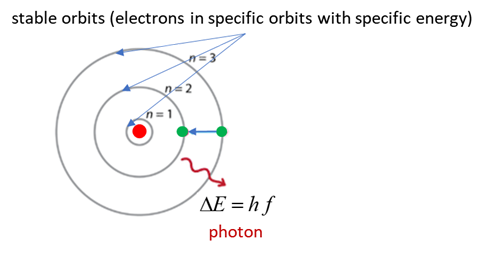
In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus.
To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump.
|