|
8.1 FROM THE UNIVERSE TO THE ATOM EARLY MODELS OF THE ATOM P81 1903 Outline the
experiment performed by Geiger and Marsden. What was the important
finding of their experiment? View the solution below only after you have completed answering the question. The solution is not in a form that you would answer in an examination. The answers are often in more detail to help improve your appreciation and understanding of the physics. |
|
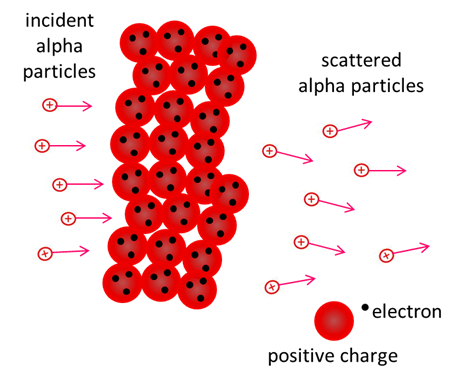
Solution Geiger and Marsden in 1911 at the suggestion of Earnest Rutherford, fired highly energetic alpha particles from the radioactive element polonium through thin metal films. Rutherford wanted to perform an experiment to test Thomson's plum pudding model. According to the Thomson's model, the energetic alpha particles would pass straight through thin metals since the positive charge and mass of the metal are uniformly distributed and so the alpha particle has little reason to swerve off its original path.
Penetration of a metal foil by alpha particles as predicted by Thomson's model. In the metal, positive charge spread uniformly and electrons embedded here and there to give weak electric fields. Therefore, very little deflection of alpha particles is expected. However, this prediction did not agree with experimental results. In the Geiger, Marsden (Rutherford) experiment, an alpha source was placed in front of a lead sheet with a hole in it to give a narrow beam. On the other side of the thin metal foil was a movable zinc sulfide screen which gave off a flash of light when struck by an alpha particle so that the trajectories of the alpha particle could be determined.
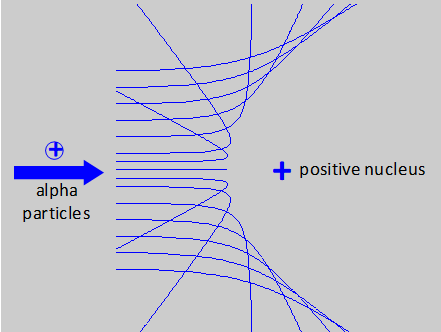
The finding of Geiger and Marsden was that, while most of the alpha particles emerged with little deflection in passing through the foil, a few particles where scattered through very large angles. Some particles were actually reflected back along their path. To Rutherford this was absolutely unbelievable: it was as incredible as if you fired a 15 inch shell at a piece of tissue paper and it came back and hit you.
The trajectories of alpha particles being scattered from a positive nucleus based upon Rutherford scattering formula. Alpha particles approaching close to the nucleus are scattered through large angles while the others pass through with little deflection. The experiments of Geiger and Marsden lead to the Rutherford model of the model. The model of the atom that emerges from Rutherford's work is a tiny, massive nucleus with a positive charge surrounded at some distance way by light electrons, with the negative charge of the electrons balancing the positive charge of the nucleus. These electrons must be in motion, otherwise the electrostatic force between them would pull the electrons into the nucleus. So, the electrons would cycle around the nucleus like the planets around the Sun in dynamically stable orbits. Therefore, the electrons must be accelerating so that they do not collapse into the nucleus. |
If you
have any feedback, comments, suggestions or corrections please email
Ian
Cooper matlabvisualphysics@gmail.com