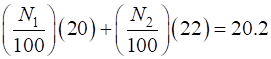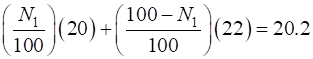|
VISUAL PHYSICS ONLINE 8.2 FROM THE UNIVERSE TO THE ATOM
THE NUCLEUS P82 004 In January
1913, J. J. Thomson reported the discovery of two types of neon atoms with
relative masses 20 and 22. (A) What name is
given to these two types of atoms? (B) The average
relative atomic mass of neon is 20.2. Calculate the proportions of the two
types of atoms in a sample of neon. View solution below only after you have completed answering the question. The solution is not in a form that you would answer in an examination. The answers are often in more detail to help improve your appreciation and understanding of the physics. |
|
Solution (A) The two types of atoms are called isotopes. Atomic number for neon is Z = 10. The two isotopes are 20Ne10 (10 protons and 10 neutrons) and 22Ne10 (10 protons and 12 neutrons). (B) Let N1 be the percentage of the abundance of the atoms with relative mass equal to 20 and N2 be the percentage for atoms with relative mass 22. Then
20Ne10 (90%) and 22Ne10 (10%)
|