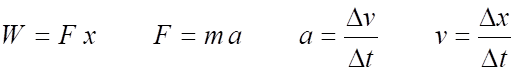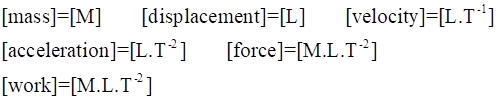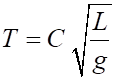|
THE S.I. SYSTEM OF UNITS
Ian Cooper email: matlabvisulaphysics@gmail.com
The system of units we have adopted for use is known as the French System International Unites. The S.I. system of units has seven base units and two supplementary units. All physical quantities may be measured in terms of S.I. base and/or supplementary units taken singly or in mathematical combinations (by multiplication and/or division). Where a unit of a physical quantity is defined in terms of such a combination of S.I. units without the use of a numerical factor or arbitrary constant is constant is called a derived unit. The base, supplementary, and derived S.I. units together form a coherent system of units, i.e. a system in which the product or quotient of any two quantities in the system is the unit of the resultant quantity, e.g. Newton’s Second Law of Motion states that the physical quantity force is related to the product of the two physical quantities mass and acceleration.
The product of the S.I. units of mass [kg] and acceleration [m.s-2] is the S.I. unit of force [kg.m.s- 2], which is given the name newton [N]. It is most important that you are familiar with the ideas and conventions of the S.I. system of units that have adopted for expressing the measurement of physical quantities.
|
|
Quantity |
SI Unit |
Symbol |
Definition |
|
Length |
metre |
m |
Length of the path of light travelled in vacuum in 1/299 792 458 of a second |
|
Mass |
kilogram |
kg |
Prototype platinum-iridium cylinder |
|
Time |
second |
s |
9 192 631 770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of cesium 100 |
|
Electric current |
ampere |
A |
Current in two infinite length conductors of negligible circular cross section, 1 m apart in vacuum that produces a force equal to 2x10-7 newtons per metre of length |
|
Temperature |
kelvin |
K |
The fraction 1/273.16 of the thermodynamics temperature of the triple point of water |
|
Amount of substance |
mole |
mol |
Amount of substance of a system which contains as many entities as there are atoms in 0.012 kg of carbon 12 |
|
Luminous intensity |
candela |
cd |
Luminous intensity, in perpendicular direction, of a surface of 1/600 000 m2 of a blackbody at the temperature of freezing platinum under a pressure of 101.325 N.m-2 |
SUPPLEMENTARY UNITS
|
Plane angle |
radian |
rad |
Radian is the plane angle between two radii of a circle that cuts off on the circumference an arc equal in length to the radius |
|
Solid angle |
steradian |
sr |
Steradian is the solid angle that has its vertex in the centre of a sphere and cuts off an area of the surface equal to that of a square with sides of length equal to the radius of the sphere |
|
PREFIX |
Pronunciation |
SYMBOL |
FACTOR |
|
yocto |
yoc'toe |
y |
10-24 |
|
zepto |
zep'toe |
z |
10-21 |
|
atto |
at'toe |
a |
10-18 |
|
femto |
fem'toe |
f |
10-15 |
|
pico |
pee'koe |
p |
10-12 |
|
nano |
nan'oe |
n |
10-9 |
|
micro |
my'kroe |
|
10-6 |
|
milli |
mil'i |
m |
10-3 |
|
centi |
sent'ti |
c |
10-2 |
|
deci |
des'i |
d |
10-1 |
|
deka |
dek'a |
da |
101 |
|
hecto |
hek'toe |
h |
102 |
|
kilo |
kil'oe |
k |
103 |
|
mega |
meg'a |
M |
106 |
|
giga |
gig'a |
G |
109 |
|
tera |
ter'a |
T |
1012 |
|
peta |
pe'ta |
P |
1015 |
|
exa |
ex'a |
E |
1018 |
|
zetta |
zet'ta |
Z |
1021 |
|
yotta |
yot'ta |
Y |
1024 |
|
The result of a measurement of a physical quantity is usually expressed as number. The number is generally meaningless without specifying its unit of measurement.
For example, measurements of people’s height may have been written as: height, h = 0. 002 meaningless because the unit is not specified height, h = 1.75 m S.I. Unit for length is the metre height, h = 1750 mm height expressed in millimetres (prefix m milli) height, h = 2.1 feet non S.I. Unit
THE USE OF PREFIXES Measurement of larger and smaller magnitude than the S.I. units are frequently made. If S.I. units only were available, numbers much greater or less then unity would be encountered. To avoid this inconvenience the SI makes provision for other units both larger and smaller than the S.I. units themselves. Rather than introducing a host of new names for these other units and setting odd numerical relationships between them and the S.I. units, the S.I. arranges for multiples and sub-multiples of the S.I. units in an orderly and consistent manner, by providing: · that the multiples and submultiples shall be decimally related to the 'parent' unit; · that the decimal relationships shall be indicated by the attachment of prefixes to the parent unit; and · that each prefix shall have a standard value regardless of the unit to which it is attached.
An anomaly exists with respect to expressions of mass. The S.I. unit is the kilogram which should be used in all calculations to take advantage of the coherent property. However, to avoid the use of two adjacent prefixes, the S.I. prefixes having their standard values are attached to the stem word 'gram' when forming units of mass larger and smaller than the kilogram. Thus, the gram itself is a sub-multiple of the kilogram.
RULES FOR USING S.I. UNITS · As a general rule, names of units and prefixes written in full are written in lower case (small) letters except at the beginning of a sentence. The sole exception is degree Celsius oC. · Unit symbols are written in lower case letters except the symbols for units named after people, when the first letter of the symbol is a capital, e.g. pascal (Pa), newton (N), ampere (A), hertz (Hz). · All prefixes except those representing a million and more i.e. mega (M), giga (G) and tera (T), have lower case symbols. · Unit names take a plural ‘s’ when associated with numbers greater than unity, e.g. 1.5 joules. The name hertz is unaltered in the plural. · Symbols are internationally recognised mathematical representations of units and are NOT abbreviations of the unit names, therefore do not take a full stop to indicate an abbreviation, nor do they take the plural form of a following ‘s’, which is the symbol for ‘second’, e.g. km NOT km. 2 kg NOT 2 kgs
· As with any other symbols, unit symbols are translated into the names when spoken, e.g. 2 kg is spoken as ‘two kilograms’ and not ‘two kay gee’. · Unit names and symbols should be separated from any associated numerical value by a space, e.g. 27 m NOT 27m 27 metres NOT 27metres The only exceptions to this rule are the locations of the symbols for degree (o) minute (') and second (") of plane angle and the symbol degrees Celsius, e.g. 274 oC. · The prefix is not separated from the parent unit, e. g. millimetre NOT miIli-metre mm NOT m m, m.m m/m · When a prefix is attached to a unit it becomes an integral part of the new unit thus created and is therefore subjected to the same mathematical processes as the parent unit, e.g. mm2 = mm x mm 0.01 m x 0.01 mm = 0.0001 mm2 NOT 0.001 m2 10-2 mm x 10-2 mm = (10-2)2 mm2 = 10-4 mm · No more than one prefix should be included in any unit, e.g. nanometre (nm) NOT millimicrometre (mmm) · Where a unit is expressed in the form of a product or a quotient the prefixed unit (if any) should be the first occurring unit, e.g. millinewton metre (mN.m) NOT newton millimetre (N.nm) · Where a unit is derived by multiplication of two or more different units, the resulting compound name is formed by stating the names of the constituent units in with a space is left between each of the successive names, e. g. newton metre · Raising of a unit to a power – power after the unit second squared (s2) mm4 millimetre to the fourth power or millimetre to the fourth placing
· By custom, the word ‘square’ or ‘cubic’ may be placed in front of a unit of length raised to the second or third, e.g. square metre, cubic metre, kilogram per cubic metre · The quotient of two units is indicated by the word ‘per’ immediately in front of the unit(s) forming the denominator. The word ‘per’ should not occur more than once in any unit name, e.g. metre per second squared · Powers of any unit are shown by the appropriate indices (superscripts) to the unit symbol, e. g. m3 ·
Products of symbols are indicated by a full stop or a
point at mid-height between the symbols, e. g. N.m, N·m · Quotients are indicated by a solidus (oblique stroke ‘/’), a horizontal line, or the use of negative indices. The solidus and the horizontal line are directly equivalent to the word ‘per’ used in unit names, so that no more than one solidus or horizontal line may be used in a unit symbol. The best way to write the unit is using indices, e.g. m.s-2 (m/s2) W.m-1.K-4 · It is best to write small and large numbers in scientific notation, e.g., 1.55x105 kg NOT 155000 kg 8.23x10-3 m NOT 0.00823 m · Commas should not be used as thousand markers since the comma is used in many countries as the decimal symbol. · Numbers having five or more figures on either side of the decimal symbol (or its position) are grouped in threes counting from the decimal symbol with a space between groups, e.g. 2 345 678 0.870 543 2 78 054.23 45 The space may be omitted where there are less than five figures on the relevant side of the decimal symbol, e.g. 5426 0.4475 10.7264 · In tabulations, it is usually desirable to include the space, e.g. 10.179 3 0.193 2 2.753 25 13.127 75 · Numbers less than unity take a zero before the decimal symbol, e.g. 0.9144
DIMENSIONS The dimensions of any physical quantity refer to the dependence of that quantity on the seven base quantities (mass, length, time, current, temperature, molar quantities and the luminous intensity).
A short hand way of stating the dimensions of a physical quantity is to use square brackets [ … ], e.g. [length] = [L] [mass] = [M] [time] = [T]
All physical quantities can be related to the above the base quantities by dimensional analysis. Consider the physical quantity work. We can calculate its dimensions as follows:
where
Therefore, the S.I. unit for force is kg.m.s-2 and this is given the special name newton (N) 1 kg.m.s-2 = 1 N
and the S.I. unit for work is kg.m2.s-2 and is given the special name joule (J) 1 kg.m2.s-2 = 1 N.m = 1 J
Dimensions can be used to check a formula. For example where
LHS
dimensions [ RHS
dimensions [
The
dimensions on the left do not match the dimensions on the right of the
equation, therefore, the equation is incorrect. The equation
Dimensions
can be used as a means of guessing how a set of physical quantities may be
related. For example, consider a simple pendulum consisting of a small object
oscillating at the bottom of a light string. We might assume the period
where C is a numerical constant. In terms of dimensions
This can only be true if
Hence, the form of the equation for the period of the pendulum is
The
value of
From 2019 there are major changes to the S.I. System of Units as outlined in the article
Revamped SI measurement system approved
The world’s system of weights and measures will soon rely totally on the values of constants rather than arbitrarily defined base units.
|