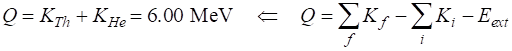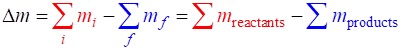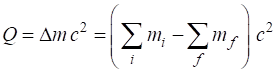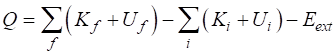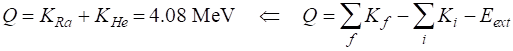|
SPECIAL RELATIVITY and NUCLEAR REACTIONS RADIOACTIVITY: ALPHA DECAY |
|
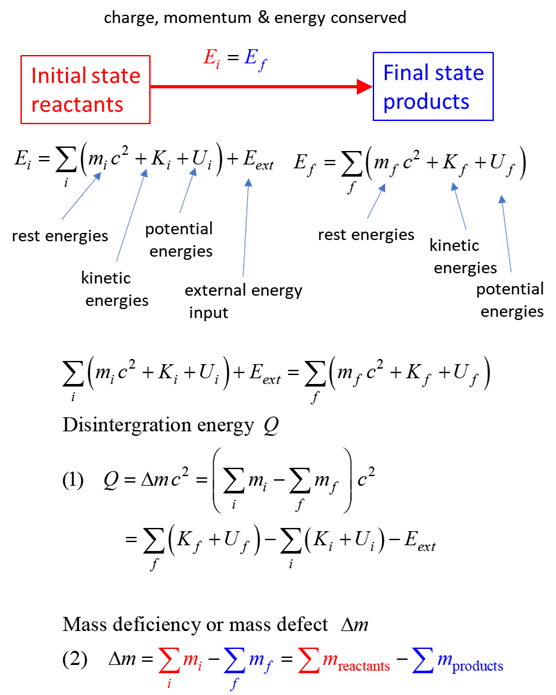
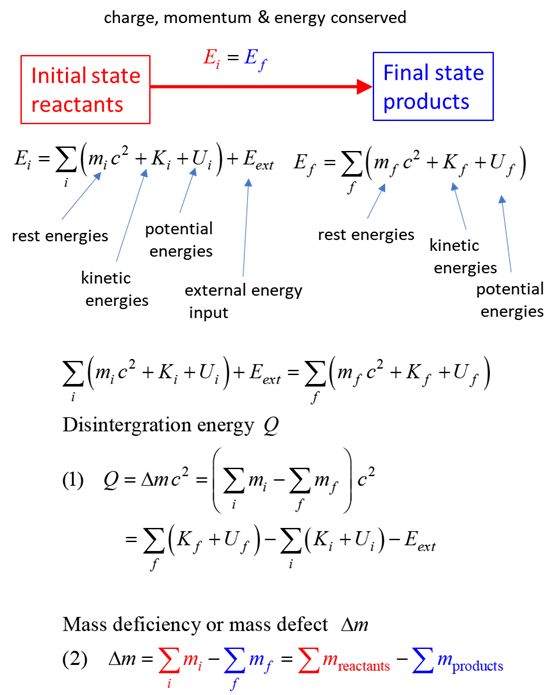
SUMMARY Total energy total
energy = rest energy + kinetic energy + potential energy
Law of
conservation mass-energy isolated
system E =
constant
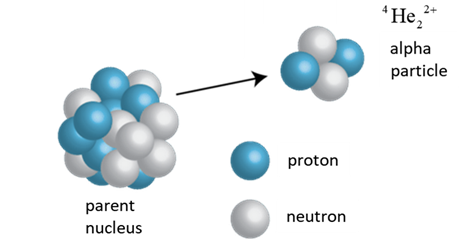
ALPHA DECAY Alpha decay is one process that
unstable atoms can use to become more stable. During alpha decay, an atom's
nucleus sheds two protons and two neutrons in a packet called an alpha
particle ( Transmutation
of a parent P into its daughter D and APZ ® A-4DZ-2 +
4He2 |
|
Energy / Mass units, values and conversion factors amu (atomic mass unit) = 1 u = 1.66054´10-27 kg 1 eV = 1.602´10-19 J 1 MeV = 106 eV A mass of 1 u
(1 amu) has an energy equivalent of: E = (1.66054´10-27) (2.99792´108)2 J = 1.49242´10-10 J E = 931.494
MeV 1 u º 931.494 MeV/c2 Proton mass mp = 1.67262´10-27 kg = 1.0072765 u = 938.3 MeV/c2
Neutron mass mn = 1.67493´10-27 kg = 1.0086649 u = 939.6 MeV/c2 Electron mass me = 9.1093897´10-31 kg = 0.0005485799 u = 0.511
MeV/c2 NUCLEAR REACTIONS
ALPHA DECAY Some isotopes are unstable and decay to form a stable,
nonradioactive nuclei. An unstable-radioactive nuclei
can emitted an alpha particle (4He2
nucleus).
An alpha
particle (a particle) is a helium nucleus 4He2
that is naturally emitted from an unstable nucleus producing a nucleus of a
new element. Emission
of a 4He2 nucleus:
N → (N – 2) Z → (Z –
2) A → (A
– 4) Transmutation of a parent P into its daughter D:
APZ ® A-4DZ-2 +
4He2
Alpha decay occurs because the
strong nuclear force is unable to hold large nuclei together (Z > 82). The
attractive strong nuclear force only acts between neighbouring nucleons since
it is short ranged. However, the repulsive electrostatic force is long ranged
and acts all the way across a nucleus and dominates the strong nuclear force.
An a particle is very a very tightly
bound unit, and therefore a helium nucleus is emitted rather than some other
combination of protons and neutrons. One widespread application of
nuclear physics is present in nearly every home in the form of an ordinary smoke detector.
Web search: How does a smoke
detector work? Alpha
particles have the least penetrating power compared to beta particles and
gamma rays as they move with a smaller velocity. Alpha particles very easily
ionize the atoms in their vicinity and hence loss energy very rapidly and
therefore doesn’t travel very far into a material. In air, alpha particles
only travel about 100 mm. Alpha
particles are not particularly dangerous to a person with external exposure. However, if ingested, they can cause serve damage to cells and
organs because of the high ionizing power. Example 1
Initial state (reactants)
radium ® radon + a
226Ra88 ® 222Rn86 + 4He2 Mass: Reactants 225.977134 u
Mass: Products 221.970399 u
4.001506 u
Mass defect dM
= 0.005229 u Disintegration value Q Q = 4.870624 MeV However, this decay is not so
simple. A gamma ray is emitted when a parent nucleus decays by emitting an
alpha particle and the daughter nucleus is left in an excited state (*). The excited daughter nucleus than
emits a gamma ray. So, in an a source,
g rays are often emitted as well as
the a particles. The excited nucleus can be represented
by the superscript *, e.g., 222Rn86* 226Ra88 ® 222Rn86 + 4He2
energy of a
particle 4.871 MeV 226Ra88 ® 222Rn86* + 4He2
energy of a
particle 4.685 MeV 222Rn86* ® 222Rn86 + g
energy of g
ray 0.186 MeV Example 2 Consider the emission of an alpha particle from the uranium
nucleus 230U92
Initial state (reactants)
230U92
mU = 230.033927 u mTh = 226.024891 u mHe = 4.002603 u
Mass deficiency Disintegration energy Alpha decay is allowed since Q
>
0. This means that the mass of the products is less than the mass of the
decaying nuclei. The energy released in the decay appears as the kinetic
energy of the thorium and helium nuclei (6.00 MeV). Example 3 We
will consider the emission of an alpha particle (helium nucleus) from a heavy
nucleus of thorium where the parent nucleus is unstable and spontaneous
explodes tearing the whole atom into two pieces.
Initial
state (reactants)
thorium ® radium + a
Mass defect
Disintegration energy
Mass: Reactants 231.988688 u
231.988688 u
Mass: Products 227.982800 u
4.001506 u
231.984306 u
Mass defect dM = 0.004382 u
Disintegration value Q Q = 4.081600 MeV The mass
defect mass becomes the kinetic energy of the products
|