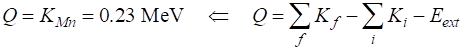|
SPECIAL RELATIVITY and NUCLEAR REACTIONS RADIOACTIVITY: BETA DECAY |
|
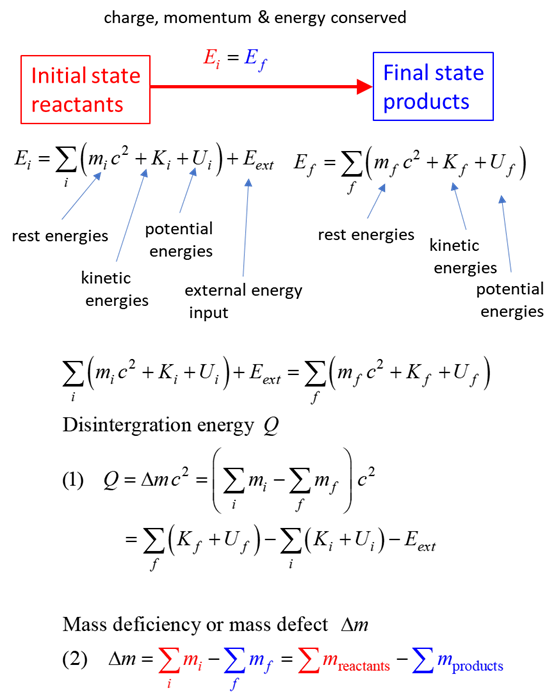
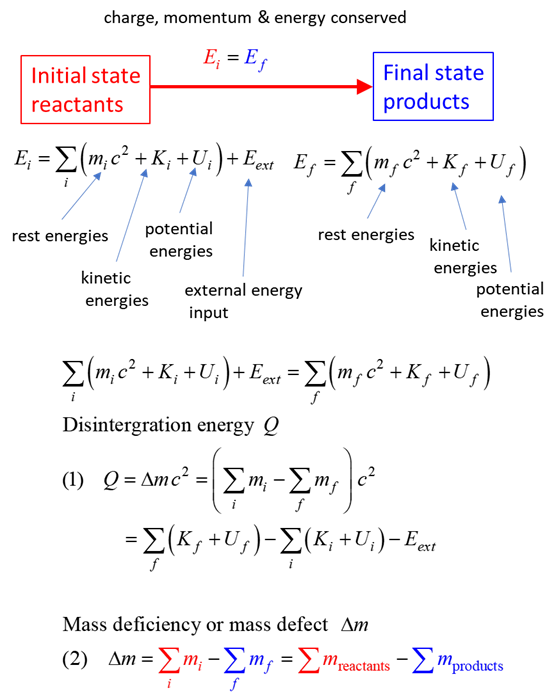
SUMMARY Total energy total
energy = rest energy + kinetic energy + potential energy
Law of
conservation mass-energy isolated
system E =
constant
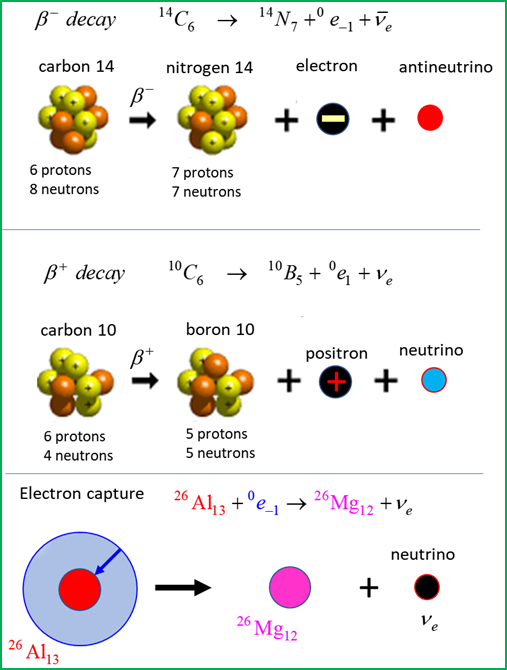
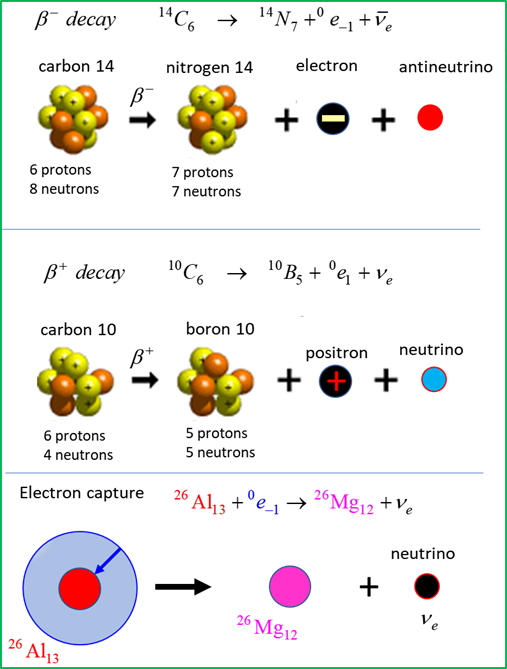
BETA DECAY Beta decay is one process that
unstable atoms can use to become more stable. There are two types of beta
decay, beta-minus
an antineutrino
neutrino Electron capture is one
process that unstable atoms can use to become more stable. During electron
capture, an electron in an atom's inner shell is drawn into the nucleus where
it combines with a proton, forming a neutron and a neutrino. The neutrino is
ejected from the atom's nucleus.
|
|
Energy / Mass units, values and conversion factors amu (atomic mass unit) = 1 u = 1.66054´10-27 kg 1 eV = 1.602´10-19 J 1 MeV = 106 eV A mass of 1 u
(1 amu) has an energy equivalent of: E = (1.66054´10-27) (2.99792´108)2 J = 1.49242´10-10 J E = 931.494
MeV 1 u º 931.494 MeV/c2 Proton mass mp = 1.67262´10-27 kg = 1.0072765 u = 938.3 MeV/c2
Neutron mass mn = 1.67493´10-27 kg = 1.0086649 u = 939.6 MeV/c2 Electron mass me = 9.1093897´10-31 kg = 0.0005485799 u = 0.511
MeV/c2 NUCLEAR REACTIONS
BETA DECAY Suppose
now that a nucleus exists which has either too many or too few neutrons
relative to the number of protons present for stability. Stability can be
achieved by the conversion inside the nucleus of a proton into a neutron or a
neutron into a proton. In this transmutation: Charge
is conserved Energy
and momentum are conserved Beta rays are more penetrating than
alpha rays and move at a very high speed.
The electron indistinguishable from an orbital electron. The symbol A free neutron can also decay but not a free proton (on average
a free neutron at rest lives for ~15 minutes)
To conserve charge, the beta
particle emitted is the positron transmutation, an electron neutrino Electron capture Besides
THE NEUTRINO In beta decay, a more stable nucleus
is produced and hence in the process energy is liberated as kinetic energy of
the products. It was first envisaged that the products of beta decay were the
only the daughter nucleus and an electron. Since daughter nucleus has a mass
much larger than that of an electron it would recoil only very slowly with
small energy whereas the electron would gain most of the energy liberated.
Hence, it was expected that the emitted electrons in beta decay would have a
fixed kinetic energy in each beta decay transmutation. But it was found
experimentally that the kinetic energy of the emitted electron could have any
value from zero up to the maximum value. It was if the law of conservation of
energy was violated. Careful measurements indicated that linear and angular
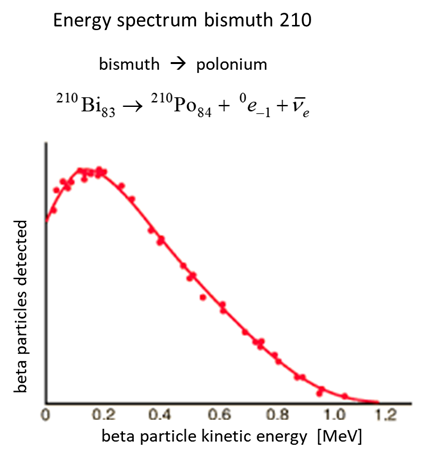
momentum were also not conserved. Figure (1) shows the energy spectrum
for the decay of bismuth into polonium.
Fig. 1. Beta decay of
bismuth-210 into polonium-210 where a neutron changes into a proton. The need to account for the energy
distribution of electrons emitted in beta decay (figure 1) and to satisfy the
laws of conservation of energy, linear and angular momentum, Austrian
physicist Wolfgang Pauli in 1930
proposed that a neutral particle was emitted along with the In 1934, Italian physicist Enrico Fermi (1901 - 1954) named Pauli’s particle the neutrino ( Despite several ingenious attempts,
the neutrino was not experimentally observed until 1956. In that year, two
American Physicists, Cowan
and Reines
successfully identified the neutrino by detecting the products of a reaction
that could only have been initiated by the neutrino. It was Fermi who, in this theory
postulated the existence of the fourth force in nature: the weak nuclear force. In beta decay, it is the weak
nuclear force that plays the crucial role. Example 1
Initial state (reactants)
Mass:
Reactants 13.999951 u
Mass: Products 13.999234 u
0.000549 u
Mass defect dM
= 0.000168 u Disintegration value Q Q = 0.156476 MeV Example 2
Initial state (reactants)
Mass: Reactants 18.996395 u
Mass: Products 18.993466 u
0.000549 u
Mass defect dM
= 0.002381 u Disintegration value Q Q = 2.217500 MeV Example 3 electron
capture
Initial state (reactants)
Mass: Reactants 7.014734 u
0.000549 u
Mass: Products 7.014358 u
Mass defect dM
= 0.000925 u Disintegration value Q Q = 0.861893 MeV Example 4 Show that the isotope 55Fe26
may undergo electron capture but not Electron capture
initial state (iron)
55Fe26 + 0e+-1
mFe = 54.938292023 u me =
5.4857990907x10-4 u
mMn = 54.938043937 u
Mass deficiency Disintegration energy Q
> 0 b+ decay
initial state (iron)
55Fe26
mFe = 54.938292023 u me =
5.4857990907x10-04 u
mMn = 54.938043937 u
Mass deficiency Disintegration energy Q
< 0 |