|
SPECIAL RELATIVITY:
Experimental Verifications NUCLEAR REACTIONS |
|
SUMMARY Total energy
total energy = rest energy + kinetic energy + potential energy
Law of
conservation mass-energy isolated system E = constant
|
|
UNITS Most calculations of mass and energy in
nuclear physics do not use S.I. units. Hence, we need to consider the units
you will often encounter. Mass
atomic mass unit 1 u =
1.66053906660×10−27 kg =
931.49410242 MeV.c-2 Energy
electron-volt
1 eV = 1.602176634×10−19 J
1 MeV = 106 eV =
1.602176634×10−13 J CALCULATIONS FOR NUCLEAR REACTIONS The mass
and energies involved in nuclear reaction, perhaps provides the most direct
confirmation of the law of conservation mass-energy and the equivalence of
mass and energy. We can set up a mathematical model based upon the law of
conservation of mass-energy that can be used for nearly all the nuclear
reaction calculations you may encounter.
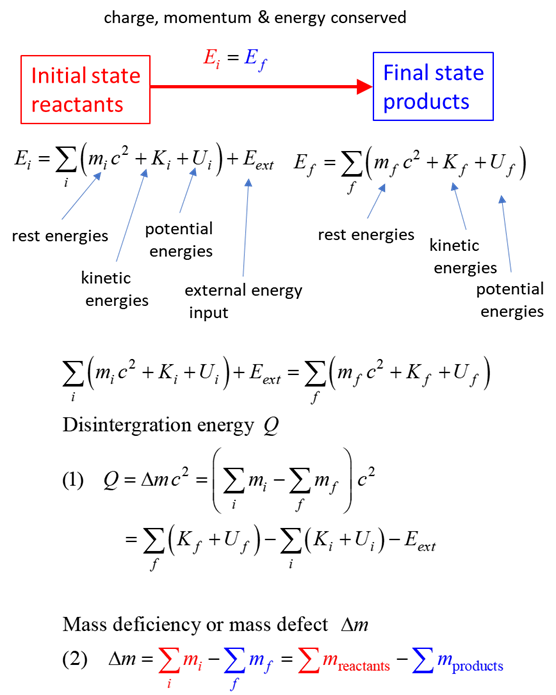
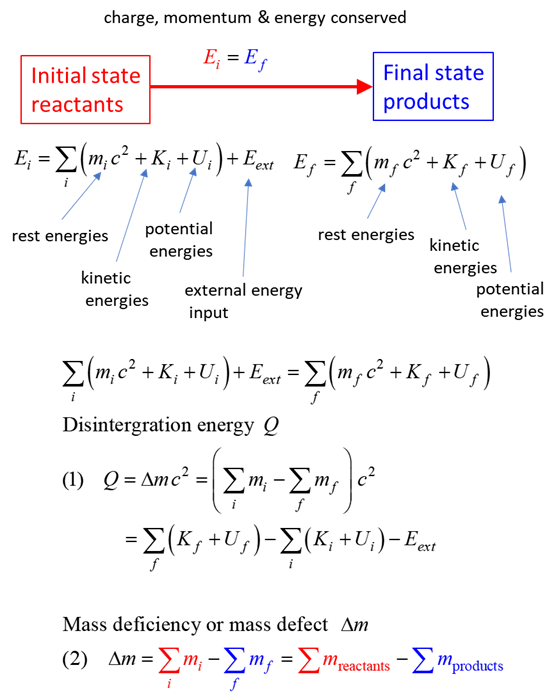
The nuclear reaction is summarized by the transformation of an initial state (reactants)
to a final state (products).
The Q-value (disintegration energy) for a reaction is the amount of
energy absorbed A reaction with a negative Q-value A reaction with a positive Q-value |
|
|