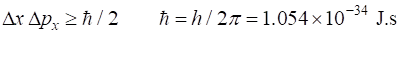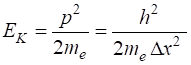|
8.1 FROM THE UNIVERSE
TO THE ATOM WHY DO ATOMS EXIST? OUR MYSTERIOUS QUANTUM UNIVERSE Have you ever thought
about why our universe exists as it does, and that the building blocks of just about everything is made of atoms. Our
model of an atom is that is made of a positive nucleus and surrounded by
negative electrons. But common sense and the laws of classical physics tells
us that a negative object is attracted to a positive object. So, the negative
electrons should just rush and collapse into the nucleus atoms should not
exist, and the world we live in would not be as we know it
!!! We can only explain
the existence of our universe and that atoms are real using the very
strange ideas of quantum physics. Do you know what a
wave is and what is a particle?
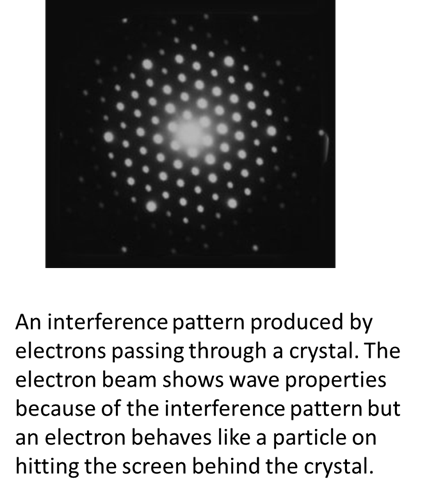
But, what about an
electron? Is it a particle or a
wave? The strange answer is that is not a classical particle or a classical
wave. The electron exhibits both particle-like and wave-like behaviour but it
is not a classical particle or a classical wave.
So, we dont know
what an electron is and cant use a picture to represent it. However, the
behaviour of electrons can be described extremely accurately using the mathematics
of quantum mechanics where predictions of their behaviour are expressed in
terms of probabilities. Hence, it is not possible to predict exactly what
will happen in any circumstance. For example, we cant predict when an
excited atom will emit light or do we know when a given radioactive nucleus
will decay. So, what happens can only be described statistically. Quantum mechanics
has many aspects. For example, the idea that a particle has a definite
location and a definite speed is no longer allowed it is wrong. An
important principle of quantum mechanics is called the Heisenberg
Uncertainty Principle that says that one cannot know where something is and how
fast it is moving at any instance of time. One way of expressing this
principle is where Why are atoms so big? Why is the nucleus so small, so massive and at the centre of the atom
with the electrons around it? Size of an atom ~ 10-10 m Size of nucleus ~ 10-15 m Volume of atom / volume nucleus ~ 1015 (an atom is really big compare with nucleus) What keeps the electrons from falling into the nucleus? If an electron was
within the nucleus, then we would know its position very accurately
Therefore, the
uncertainty principle contains implications about the energy that would be
required to contain a particle within a given volume. The value of Planck's
constant h, appearing in the
uncertainty principle, determines the size of the confinement that can be
produced by the fundamental forces of nature. The following very approximate
calculation serves to give an order of magnitude for the energies required to
contain electrons within an atom and nucleus.
Energy to contain an electron with an atom Assume: Location Momentum Kinetic energy Uncertainty principle So, binding energies of
electrons in atoms and energies involved in chemical reaction are in the
order of a few electron volts. Energy to contain an electron with a nucleus Assume: Location Momentum Kinetic energy Uncertainty principle The energy to confine an
electron to the nucleus is more than a thousand greater than the energies
observed in nuclear processes. So, electrons cannot be confined within a
nucleus. If you repeat the calculation for a proton confined to a nucleus you get a result of the confinement energy of 2 MeV. Energies observed for nuclear processes are in the order of a few MeV. Hence, protons and neutrons can be confined with the small volume of a nucleus by the strong nuclear force. |