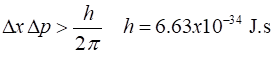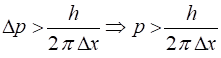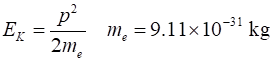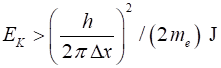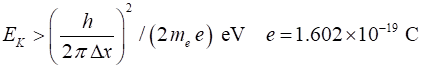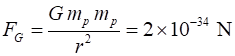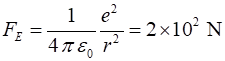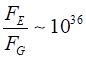|
THE NUCLEUS AND THE STRONG NUCLEAR FORCE MODELS OF THE ATOM J. J. Thomson
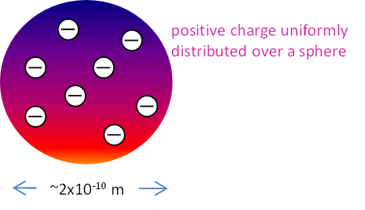
model of the atom
(1907) plum-pudding
model:
positive charge uniformly distributed over a sphere of radius ~10-10
m with the electrons spread out throughout the sphere in such a way that the whole
system is stable and electrically neutral
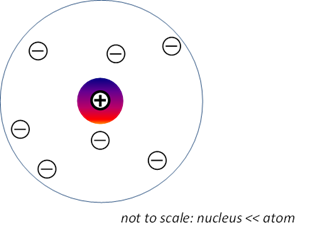
Rutherford model
of the atom (1911) Atom consists of a very tiny but positively
charged nucleus containing over 99.9% of the atoms mass, surrounded by
electrons some distance away. The electrons would be moving in orbits about
the nucleus, much as planets move around the Sun.
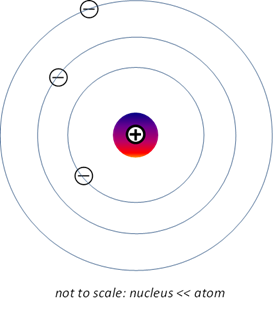
Bohr-Rutherford
model of the atom (1913) Bohr proposed a planetary model of the atom
based upon the Rutherford model but added the restriction that the electrons
can only orbit the nucleus in circles but only with certain radii allowed
such that the angular momentum of the electron is quantized.
atomic dimensions ~ 10-10
m nuclear dimensions ~
10-15 m THE NUCLEUS The nucleus of an atom is a tiny positively charged lump which
contributes to the majority of the mass of an atom
and holds its electrons in place. But, it is the atomic electrons that are
responsible for the characteristics and behaviour of matter in bulk and not
the nucleus. What is the nucleus made of? In the periodic table, the elements are listed in order of their
atomic number Z, with the number of the element
defined as the number of protons within a nucleus. 1 hydrogen 2 helium 92
uranium The atomic mass of the elements increases with atomic number Z
and it was first suggested that all atoms are simply combinations of hydrogen
atoms (protons). Thus, a helium atom (Z = 2) should have a nucleus with two
protons, a lithium atom (Z = 3) should have a nucleus composed of 3
protons, and so on. However, atomic masses do not increase in steps of one
hydrogen atom mass. Helium atoms weigh about 4 times as much as hydrogen
atoms and lithium atoms about 7 times as much as hydrogen atoms. But, atomic
masses were very close to exact multiples of the mass of the hydrogen atom! The Proton-Electron Model It was hypothesized that there are enough protons in each
nucleus to provide for the observed atomic mass, with several electrons
present whose negative charge would cancel out the excess positive charge
of the extra protons. However, this is not an acceptable model of the nucleus
since too much energy would be required to localize electrons within the
nucleus according to the Heisenberg Uncertainty Principle. Rutherford (1914): an atom such as fluorine (atomic number 9)
for example, had a mass equivalent to 19 protons but a charge of only 9
protons the nucleus contained protons and electrons to balance the
charge discrepancy. A fluorine nucleus
would therefore contain 19 protons and 10 electrons a total charge of 9
protons and total mass of 19 protons (electron mass being negligible compared
to the proton mass) a nucleus contained A protons and (A Z) electrons. This model could explain how a and b particles could be
emitted from some radioactive nuclei but problems
arose: Energies of emitted b particles could not be accurately predicted. Quantum number anomalies arose with the spin of electrons and protons
within the nucleus. Heisenbergs Uncertainty Principle suggested that electrons
could not be confined within the nucleus. Uncertainty
Principle Uncertainty in
position of electron
~ size of nucleus Uncertainty in
momentum and hence minimum value of momentum of electron Minimum kinetic
energy of electron Energies of electrons in atoms are ~ eV and so electrons cant
exist in the nucleus with enormous energies > 1010 eV. Electrons are not
a constituent of a nucleus The Neutron Rutherford (1920): a proton and an electron within the nucleus
might combine to produce a neutral particle.
He named this particle the neutron. Experimental difficulties associated with
the detection of a neutral particle greatly hindered the research. In 12 years of searching, no such particle
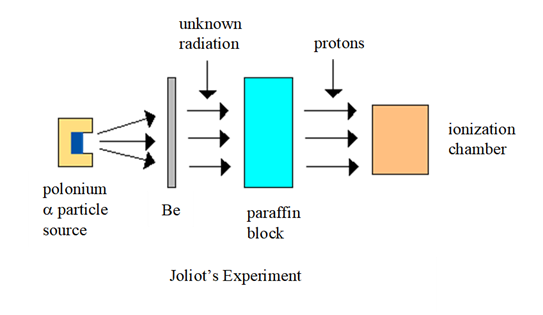
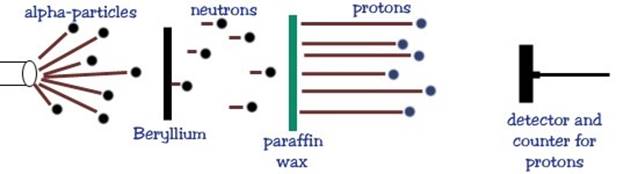
was found. In 1930, two German physicists, Bothe & Becker, bombarded
the element beryllium Be with a particles and found a very penetrating form of radiation that
was much more energetic than gamma-rays was emitted from Be. Frederic & Irene Joliot (daughter of Marie Curie) in 1932:
although this radiation could pass through thick sheets of lead, it was
stopped by water or paraffin wax. They
found that large numbers of very energetic protons were emitted from the
paraffin when it absorbed the radiation. The Joliots assumed that the radiation
must be an extremely energetic form of gamma radiation.
The English physicist, James Chadwick (1932)
showed theoretically that gamma rays produced by a particle bombardment of Be would not have enough energy to
knock protons out of paraffin, and that momentum could not be conserved in
such a collision between a gamma ray and a proton. Chadwick repeated the
Joliots experiments many times. He
measured the energy of the radiation emitted from the Be and the energies
(and therefore the velocities) of the protons coming from the paraffin. Based on its great penetrating power,
Chadwick proposed that the radiation emitted from the Be was a new type of
neutral particle the neutron, as originally proposed by
Rutherford.
He then applied the conservation of
energy and momentum laws to his experimental results and showed that the particles
emitted from the Be had to be neutral with about the same mass as the proton. Chadwick had indeed discovered the neutron. Chadwick
explained that when the neutrons emitted from the Be collided with the light
hydrogen nuclei in the paraffin, the neutron came to a sudden stop and the proton
moved off with the same momentum as the neutron had before the collision. 4He2 + 9Be4 12C6 + 1n0
The Proton-Neutron
Model Following Chadwicks discovery of the neutron, a new model of
the nucleus was proposed. This model
suggests that the nucleus consists of protons and neutrons. Together these particles are called the nucleons
, the particles that make up the nucleus. nucleon is a generic term for a proton
or a neutron Nuclear masses Z Proton number (Atomic Number) element N Neutron number A Mass number A = Z + N Isotopes: nuclei with the same atomic number Z Isobars: nuclei with the same mass number A The number of protons in the nucleus is called the atomic number Z of the nucleus and corresponds to the position of the
nucleus in the Periodic Table of Elements. For example: hydrogen 1H1 2H1
(deuterium) 3H1 (tritium) carbon 12C6 13C6 14C6 Nuclear Radius We cant talk about the definite size of a nucleus because of
the wave-particle duality principle. However,we
can think about the nucleus as a fuzzy ball whose spatial extent can be
measured by scattering high speed electrons off nuclei. The approximate
radius R of a nucleus is found to
increase in size with mass number A as
given by R = Ro A1/3 A
is mass number (number of nucleons) Ro = 1.210-15
m = 1.2 fm
1 fm = 10-15 m fm =
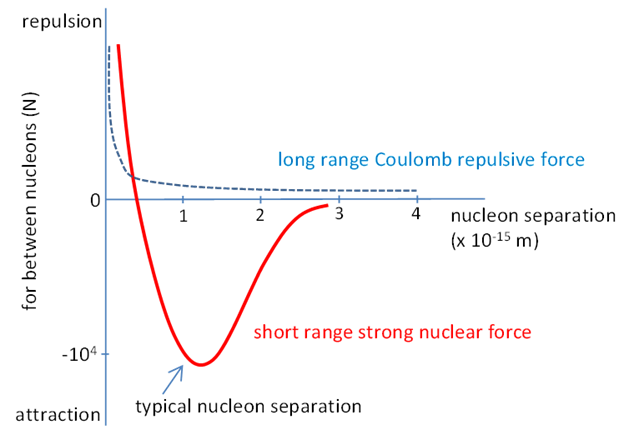
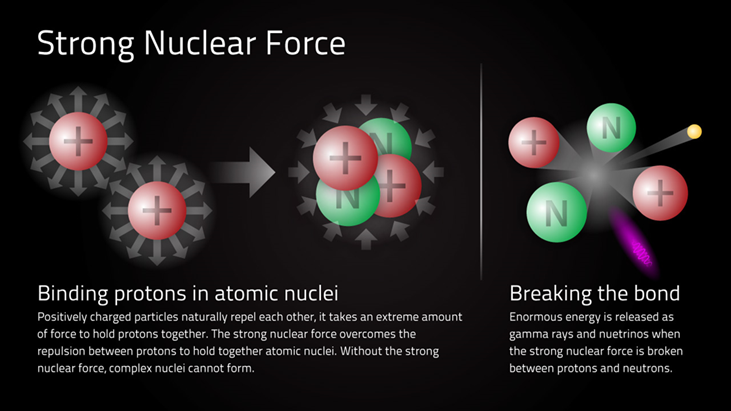
femtometre or fermi STRONG NUCLEAR FORCE Does not depend on charge i.e. binding is the same for protons
and neutrons. It has very short range ~10-15 m. A nucleon only
interacts with neighbouring nucleons (saturation of nuclear force). Nuclear force favours binding of pairs of protons or neutrons
with opposite spin. The force is really between quarks as three quarks
combine to give either a proton or neutron. Nature of NUCLEAR FORCE is not well understood.
Fig.
1. Strong nuclear force between pairs
of nucleons. A nucleus is composed of a collection of protons and neutrons,
but the protons are positively charged and so there is a very large repulsive
force between them when they are close together. Consider two protons with a separation distance r r = 1 fm = 1x10-15 m nuclear dimension mp = 1.67262x10-27 kg qp =
+1.602x10-19 C Gravitational force between two protons Electrostatic force between two protons Ratio Use your
calculator to check the numerical values Even at this very close distance the gravitational force between
protons is negligible and the magnitude of the electrostatic force is
enormous. The strong
nuclear force is an attractive force that acts between all
nucleons (protons and neutrons) and at short distances (nuclear dimensions)
is greater in magnitude than the electrostatic force acting between the
protons. The nuclear force is a much more complicated force than either the
electromagnetic force or gravitational force. The strong nuclear force is
very strong between a pair of nucleons only if their separation distance is
less than 10-15, for distances greater than this, the force is
essentially zero. If a nucleus contains too many or too few neutrons relative to
the number of protons, the binding of the nucleus is reduced, and the nucleus
is unstable, and the nucleus will decay into a more stable one (radioactive
decay). Nuclei with A < 40 tend to be stable when the number of protons
equals the number of neutrons. When A > 40, the stable nuclei have more
neutrons than protons because the increasing number of protons in a nucleus
increases the electrostatic repulsive force acting between, making the nuclei
more unstable. When Z > 82, there are no completely stable nuclei. There is also a second type of nuclear force, which is called
the weak nuclear force,
and is much weaker in strength than the strong nuclear force. The weak
nuclear force is responsible for certain types of radioactive decay known as beta (b) decay. These two nuclear forces, the strong and the weak, together with
the electromagnetic and gravitational forces, comprise the four
known types of forces acting in nature. Relative strengths of the four fundamental forces of nature Strong nuclear force 1
short range ~ 10-15
m Electromagnetic
force 1/137
charged particles inverse square law
infinite range Weak nuclear force
10-6 short range ~ 10-18 m Gravitational
force 10-40
mass inverse square law
weakest force, infinite range
|
If you have any feedback, comments,
suggestions or corrections please email:
Ian Cooper School of Physics University of Sydney
ian.cooper@sydney.edu.au