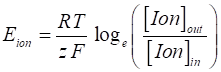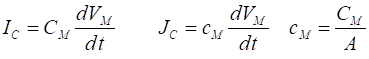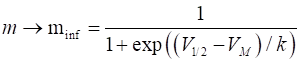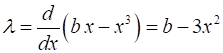|
NEURONES AS NONLINEAR DYNAMICAL SYSTEMS Neurone Models for [1D] Dynamical Systems Ian Cooper Email: matlabvisualphysics@gmail.com |
|
MATLAB SCRIPTS np002B.m The function ode45 is
used to solve the equation of motion to calculate the time evolution of the
membrane potential for different models.
To obtain the plots in this article, the Script needs to be modified
by commenting and uncommenting the close all and hold on statements and by changing the plot parameters.
The Script models the nonlinear dynamical system for a leak current / fast
sodium ion current to simulate the upstroke of an action potential. For the
leak current model, let GNa = 0 (zero sodium conductance). np001.m Simplified version of Script np002B.m for leak current only. Comparison of numerical and
analytical solutions. np001A.m Simplified versions of Script np002B.m for leak current / sodium ion current with a constant
sodium conductance. np003.m Script can
be used to give the phase portrait plot and time evolution of the state
variable x
for systems of the form Cell1: Phase Portrait Plot: Define function in Cell 1 Cell 2: Time Evolution of State Variable:
Solve equation of motion Cell 3: Define function in Cell 3 at end of
Script Different functions can be modelled by changing
the Script in Cells 1 and 3. Includes code for finding the zero of a
function. np003A.m An extension of Script np003.m for systems of the form |
|
INTRODUCTION This document is based upon the book by
Eugene M. Izhikevich Dynamical Systems in Neuroscience: The Geometry of Excitability and
Bursting This book is devoted to a systematic study of
the relationship between electrophysiology, bifurcations, and computational
properties of neurones. Information processing depends not only on the
electrophysiological properties of neurones but also on their dynamical
properties. Even if two neurones in the same region of the nervous system
possess similar electrophysiological features, they may respond to the same
synaptic input in very different manners because of each cell’s
bifurcation dynamics. Electrical activity in neurones is sustained
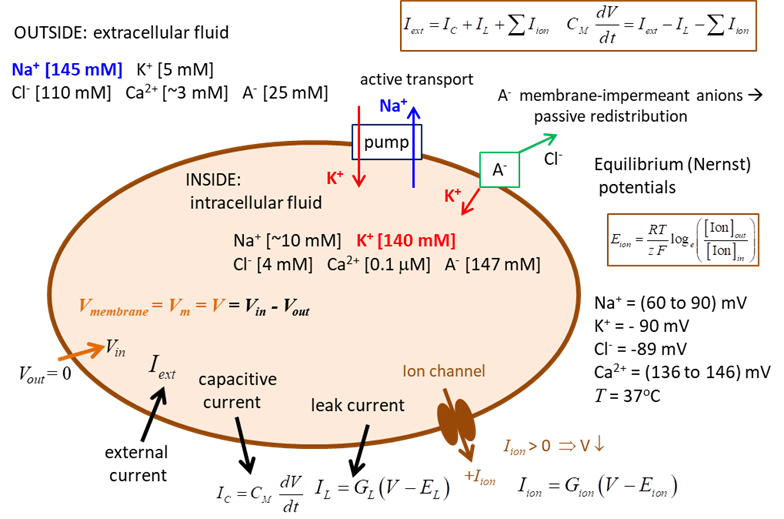
and propagated by ion currents through neurone membranes as shown in figure 1.
Most of these transmembrane currents involve four
ionic species: sodium Na+, potassium K+, calcium Ca2+
and chloride (Cl-). The concentrations
of these ions are different on the inside and outside of a cell. This creates
the electrochemical gradients which are the major driving forces of neural
activity. The extracellular
medium has high concentration of Na+ and Cl-
and a relatively high concentration of Ca2+. The intracellular medium has high concentration of K+
and negatively charged large molecules A-. The cell membrane has
large protein molecules forming ion channels through
which ions (but not A-) can flow according to their
electrochemical gradients. The concentration asymmetry is maintained
through ·
Passive redistribution: The impermeable anions A-
attract more K+ into the cell and repel more Cl-
out of the cell. ·
Active transport: Ions are pumped in and out of
the cell by ionic pumps. For example, the Na+/K+ pump,
which pumps out three Na+ ions for every two K+ ions
pumped.
Fig. 1. Electrophysiology of a neurone. Nernst or Equilibrium Potential There are two forces that drive each ion
species through the membrane channel. (1)
Concentration gradient: ions diffuse down the
concentration gradient. For example, the K+ ions diffuse out of
the cell because K+ concentration inside is
higher than outside. (2)
Electric potential gradient: as ions diffuse across the
membrane a charge imbalance occurs producing a potential difference between
the inside and outside of the cell. For the K+ ions exiting the
cell, they carry positive charge with them and leave a net negative charge
inside the cell (consisting mostly of impermeable anions A-),
thereby producing the outward K+ current. The positive and negative charges accumulate
on the opposite sides of the membrane surface creating an electric potential
gradient across the membrane. This potential difference is called the transmembrane potential or membrane voltage (1) where the extracellular potential is
the reference potential such that This potential slows down the diffusion of K+,
since K+ ions are attracted to the negatively charged interior and
repelled from the positively charged exterior of the membrane. At some point,
an equilibrium is achieved. When the concentration
gradient and the electric potential gradient exert equal and opposite forces
on the ions, the net cross-membrane current is zero. The value of such an equilibrium potential depends on the ionic species and
it is given by the Nernst equation (2) where [Ion]in
and [Ion]out are concentrations of the ions inside and outside the
cell respectively, R is the universal gas constant (R = 8.3155 J.mol-1.K-1),
T is temperature in degrees Kelvin, F is Faraday’s (F = (96 485 C.mol-1), z is the valence of the ion (z = 1 for Na+ and K+,
z = -1 for Cl-, and z = 2 for Ca2+). Eion is also called the reversal potential.
Fig. 2. Diffusion of K+ ions
down the concentration creates an increasing electric force directed in the
direction opposite to the force due to the concentration difference until the
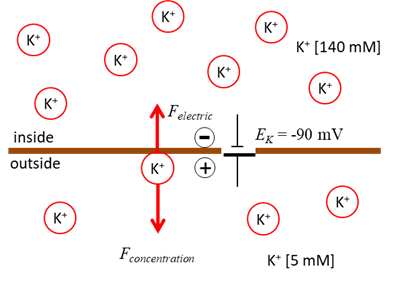
diffusion and electrical forces balance each other. Membrane Currents We can model the movement of ions
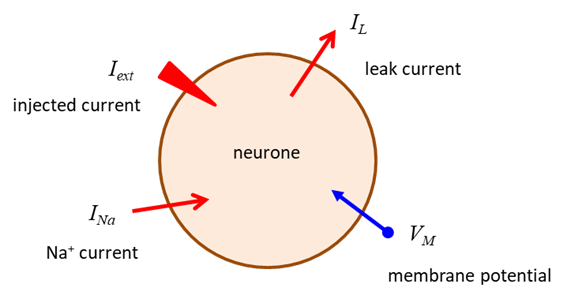
across the membrane as an electric circuit as shown in figure 3.
Fig. 3. Equivalent circuit
representation of a nerve cell membrane. In the neuroscience literature,
there is often some confusion and inconsistencies in the use of scientific
language and the units used for physical quantities. For example, the terms
conductance and conductance per unit area are often not distinguished and g
maybe the conductance or conductance per unit area with units S or S.cm-2.
In Izhikevich’s book, he gives the current in In the Scripts to model the
dynamic behaviour of neurones, S.I. units are used for all input parameters
and calculations. However, results may be expressed in non S.I. units, for
example, mV for voltage. The current through a resistive
element can be expressed by the equation
(3)
I current [ampere A] V potential difference [volts V] R resistance [ohm G conductance [siemens S 1 S This equation can also can also be
expressed in expressed in terms of the current density
(4)
A area [m] J current
density [A.m-2] g specific conductance [S.m-2] The major ion currents shown in
figure 2, can be expressed as
(5) X
ion (e.g. Na+ K+ leakage) VM membrane
potential (voltage) [volt V] EX
equilibrium (Nernst) potential
[V] When the conductance is constant,
the current is said to be ohmic.
In general, ionic currents in neurones are not ohmic, since the conductance
may depend upon time, membrane potential, and
pharmacological agents (e.g. neurotransmitters). It is the time-dependent
variation of conductances that allow a neurone to generate an action
potential (spike). The membrane acts like a capacitor
– an insulator (membrane) surrounded by the extracellular and
intracellular fluids (conductive plates). When the membrane potential
changes, a current is generated to charge or discharge the capacitor. The
capacitor current is given by the time derivative of the voltage
(6) CM
capacitance [F] cM specific
capacitance [F.m-2] The equivalent circuit to
represent the electrical properties of membranes is shown in figure 3.
According to Kirchhoff’s Current Law, the sum of the currents entering
and leaving a junction must add to zero. Hence across the membrane (7) Therefore, we can write an
“equation of motion” to describe the dynamical system of a
neurone as
(8) Note:
The membrane potential is
typically bounded by the equilibrium potentials
One example of the application of
equation (8) is the Hodgkin-Huxley
Model. For different models of a neurone, equation
(8) can be solved using the Matlab ordinary differential equation solver ode45. GEOMETRICAL METHODS OF ANALYSIS OF [1D] DYNAMICAL SYSTEMS In this section will start our
study of the geometrical methods of analysis of [1D] dynamical systems, that
is, systems having only one variable. This section is linked to Chapter 3 of Izhikevich’s book. However, the results given in
this chapter using Izhikevich’s input data could
not be reproduced using my Scripts. I had to change the numerical values used
for the simulations. In general, [1D] dynamical systems
are described by ordinary differential equations of the form
where V is a scalar time-dependent
variable which represents the state of the system. V is called a state variable. Since Space-clamped membrane having only a ohmic leak current A space-claimed membrane is one in
which the membrane potential is fixed by an experimenter and an external current
exerted into the neurone. The space-clamped condition is Possibly, the simplest [1D]
dynamical system we can look at is a neurone which has only an ohmic leak
current. The equation of motion to be solved is (9A) (9B) (9C) The membrane potential VM is the single time-dependent
variable. The membrane capacitance CM, the leak conductance GL, and the leak reversal potential EL are all constants. The explicit analytical solution
of equation 9C is (9D) where the initial value of the membrane
potential is Equation 9C is solved numerically
and analytically using the Script np001.m.
using the input parameters: % conductance [19e-3 S] G = 19e-3; % Membrane capacitance [10e-6
F] C = 10e-6; % Reversal potential / Nernst
potential [EL = -67e-3 V) E = -67e-3; % Simulation time [5] N = 10; % Initial membrane
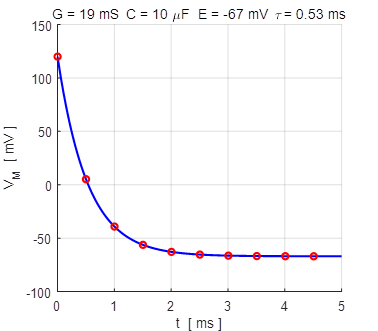
voltage [0 V] V0 = 20e-3; The equation of motion, equation 9C is solved using the ode45 function The Script can be run for different initial values of the membrane potential Vo and one can compare the numerical and analytical solutions as shown in figures 4A and 4B.
Fig. 4A. The membrane potential as a
function of time for two different initial values of the membrane potential.
The red dots show the analytical
solution and the blue curve, the numerical solution. np001.m
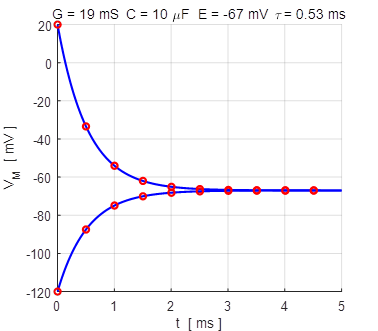
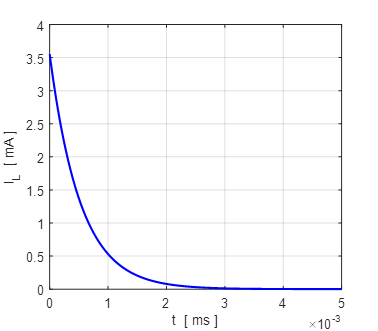
Fig.
4B. The membrane currents
as function of time for two different initial values of the membrane
potential. A
phase portrait is a geometric representation of the trajectories of a
dynamical system in the phase plane. Each set of initial conditions is
represented by a different curve, or point. Phase portraits are an invaluable
tool in studying dynamical systems. The phase portrait for our neurone model
is a plot of the time derivative of the potential against the potential
(figure 5) is a straight line. For all initial conditions, the membrane
potential will be pulled to the reversal potential
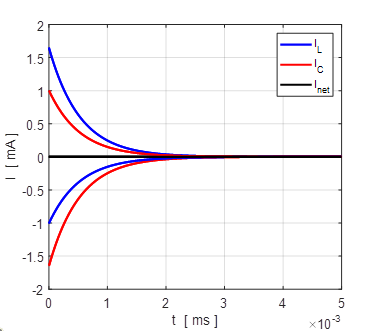
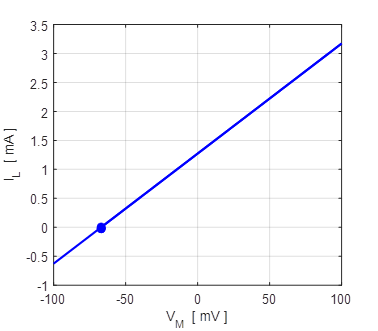
Fig. 5A. The straight line corresponds to the phase portrait plot for an ohmic element. The blue dot shows the reversal potential when time derivative of the potential becomes zero. np001.m Also, a useful plot is the I-V characteristic curve as shown in figure 5B.
Fig. 5B. I-V characteristic curve for two different initial conditions (-100 mV and +100 mV). The blue dot represents the equilibrium point (stable equilibrium) where the final current is zero and the membrane potential equals the leak reversal potential. A positive value of the current indicates an outward current to decrease the membrane potential while a negative current is an inward current which increases the membrane potential. The straight means that the conductance is constant and the slope of the line gives the numerical value of the conductance (G = 19 mS). In our simple model, the steady-state solution of the equation of motion 9C is (10) The
point
Fig.6. The initial membrane voltage is reduced to the leak reversal potential (steady-state) by a net outward current (net movement of positive charge from inside to outside the cell membrane) that hyperpolarizes the membrane. If the initial value of the membrane potential was less than the reversal potential, then a net inward current would depolarize the membrane. In
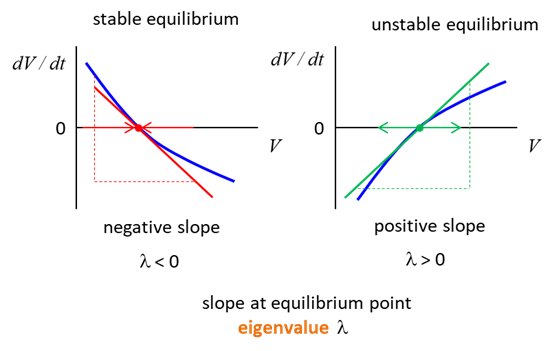
the qualitative analysis of any dynamical system it is important to find the
equilibria or rest points, i.e., the values of the state variable where F(V) = 0 and V
is an equilibrium value. At each such point We can add to our model a sodium ion channel which has a constant conductance. The equation of motion for our model is (11) Equation (11) is solved using the Script np001A.m with input parameters % conductance
[19e-3 74e-3 S] GL = 19e-3; GNa = 74e-3; % Membrane
capacitance [10e-6] C = 10e-6; % Reverse potential
/ Nernst potential [EL = -67e-3
V ENa
= 60e-3) EL = -67e-3; ENa =
60e-3; % Simulation
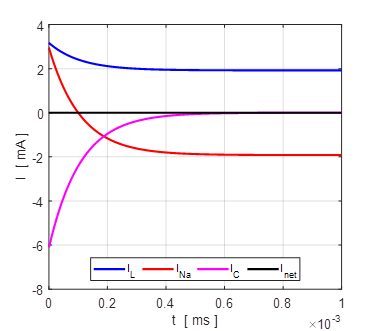
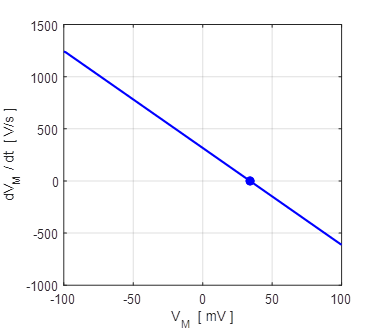
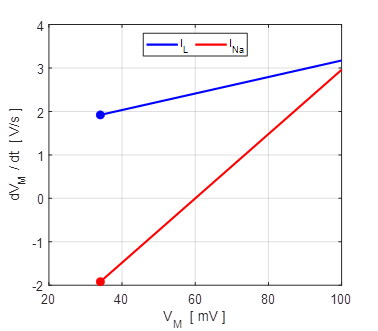
time [13-3 s] tMax = 1e-3; The results are displayed in figures 7, 8 and 9.
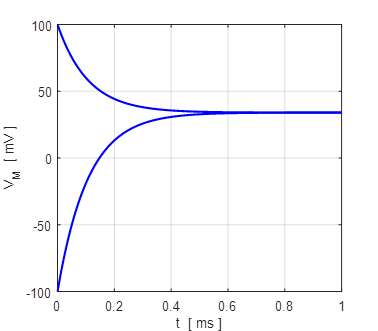
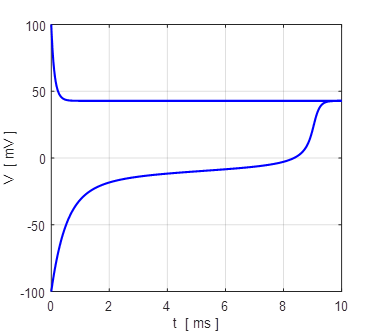
Fig. 7. The membrane potential VM evolves to its
equilibrium value of +34 mV from an initial value of +100 mV and -100 mV.
Fig. 8. The time evolution of the membrane currents. At equilibrium, the outward leakage current is balanced by the inward Na+ current. Initial membrane potential is +100 mV. np001A.m
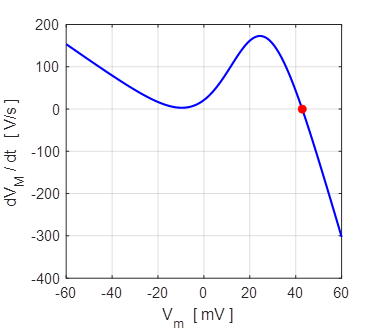
Fig. 9A. Phase portrait plot. The stable equilibrium point is (34, 0). np001A.m The I-V characteristic plot is shown in figure 9B for the leak and sodium ion currents.
Fig. 9B. I-V characteristic plot for the leak current (blue) and sodium current (red). When a steady-state is reached (shown by the two dots), the outward flow of the leak current is balanced by the inflow of sodium ions. Space-clamped membrane having only a ohmic leak current and sodium ion
channel: Leak / fast Na+ model
We can extend our simple model further my including
a voltage dependent sodium ion channel and not an ohmic Na+
channel. The conductance of an ion channel is time and voltage sensitive. We
will consider a space-clamped membrane having a leak current and a fast
voltage-gated sodium ion current having only one variable m. The gating process is assumed to be instantaneous
such that the variable m is equal to
its asymptotic value minf where (11)
Fig. 10.
The activation function The dynamics
of the system is described by the equation (12) The Script np002B.m can be used to solve equation 12 using the ode45
function. Model parameters can be changed in the input section of the Script.
Typical values are: % INPUTS
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> % conductance [19e-3 74e-3 S] gL = 19e-3; gNa = 74e-3; % Membrane capacitance
[10e-6] C = 10e-6; % Reverse potential / Nerest potential
[EL = -67e-3 V ENa = 60e-3] EL = -67e-3; ENa
= 60e-3; % Simulation time [5e-3 s] tMax =
10e-3; % Initial membrane
voltage [0 V] unstable equilibrium 6.672903e-3 V0 = 6.5672903e-3; % V1/2 [V] k [V] Vh = 19e-3;
k = 9e-3; % External current input [A] Iext =
0.60e-3; For certain input parameters, there is a
“negative conductance” and our model can show a few interesting
nonlinear phenomena, such as bistability
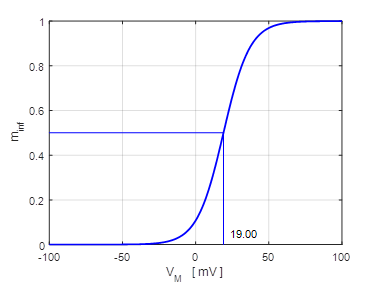
(co-existence of the resting state and excited state). The phase portrait
plot for the solution to equation 12 for different initial conditions is
shown in figure 11 which shows three distinct equilibrium points where
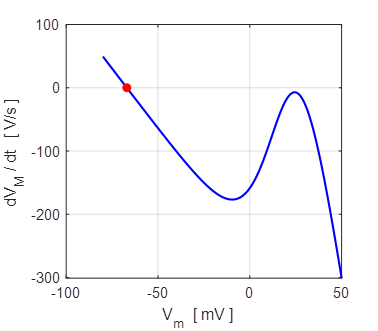
Fig.
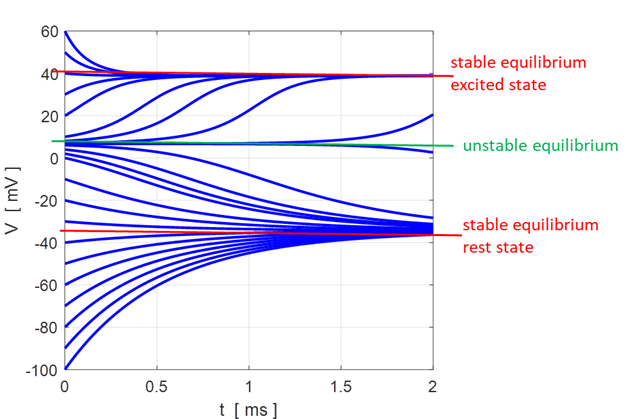
11. There are two
attraction domains which are separated by the unstable equilibrium point at
+6.6729 mV. For any initial membrane potential, the membrane potential
will converge to one of the two stable
equilibrium points: ‑ 34.5 mV (rest state) or + 38.8 mV
(excited state). The red arrows show the
attraction domains. An attraction domain of an attractor is the set of all
initial conditions that lead to the attractor. np002B.m
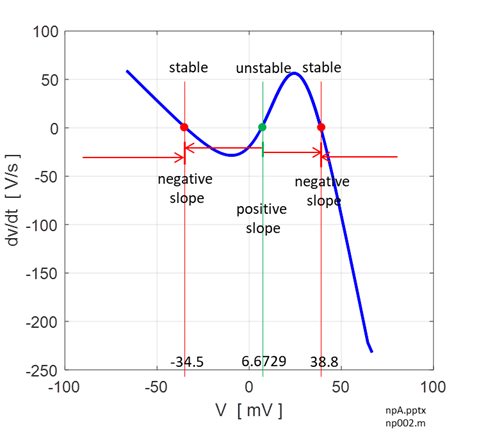
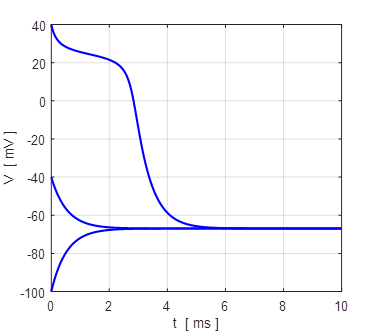
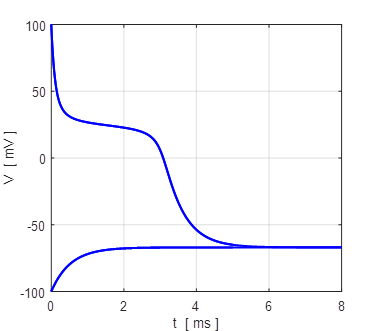
Fig. 12. Voltage trajectories for the leak / fast sodium ion model for different initial conditions. For all initial values of the membrane potential greater than the unstable membrane potential, the membrane potential is attracted to the excited state and for initial values less than the unstable equilibrium potential, the membrane potential is drawn to the resting state (bistability). The attraction to a stable equilibrium point can take a relative long time when an initial membrane potential is close to the unstable equilibrium potential. Iext = 0.60 mA np002B.m If the initial
value of the membrane potential variable is exactly at equilibrium, then If the initial value
is near equilibrium, then the membrane potential may approach or diverge from
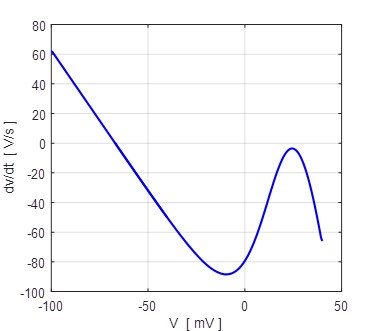
it. At an equilibrium point, when the slope of the curve At an equilibrium
point, when the slope of the curve
Fig. 12A. The slope of the curve The slope of the
curve Figure 13 shows
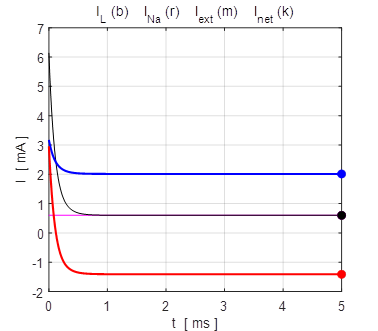
the time evolution of the leak current IL, Na+ current INa the external current Iext,
and the combined current IL
+ INa.
The upper plot is for V0 = + 100 mV and the lower plot V0 = - 100
mV. At an equilibrium point the net current or combined current is equal to
the external current
Fig. 13. The time evolution of the
membrane currents: leak current IL, Na+ current INa the external current Iext,
and the combined current IL + INa . The upper plot is for V0
= + 100 mV (stable equilibrium + 38.8 mV) and the lower plot V0 = -
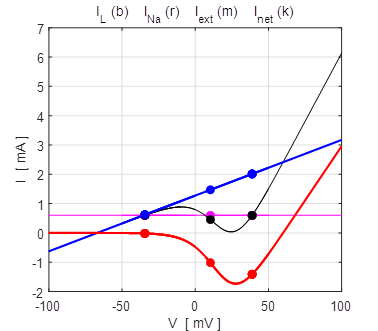
100 mV (stable equilibrium + 38.8 mV). np002.m Figure 14 shows the I-V characteristic plot: leak current IL, Na+ current INa, the external current Iext, and the combined current IL + INa. The shape I-V characteristic curve for the combined current IL + INa agrees very well with measured data from layer 5 pyramidal cells in a rat visual cortex. The slope of the I-V curve is equal to the conductance. For a region of membrane potentials, the slope has negative values and hence “negative conductance”. This negative conductance creates a positive feedback between the voltage VM and the gating variable minf (figure 10), and it plays an amplifying role in neurone dynamics. Such currents are referred to as amplifying currents.
Fig.
14. The I-V characteristic
plot: leak current IL, Na+
current INa the
external current Iext,
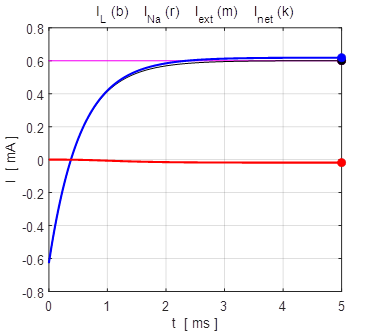
and the combined current IL + INa np002.m When the external current is set to zero, Iext = 0 A, there is only one stable equilibrium point, and the trajectory of the membrane potential is pulled a resting state.
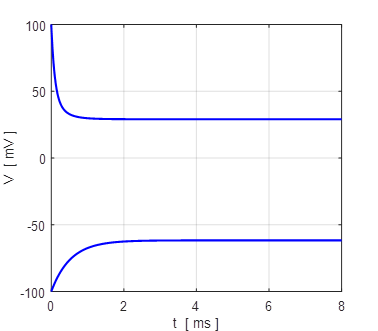
Fig. 15.
All trajectories for different initial membrane potentials are
attracted to the single stable equilibrium point, VM = - 67 mV (monostability).
Fig. 16. There is only one point where The transition
between two stable states separated by a threshold is relevant to the
mechanism of excitability and generation of action potentials of many
neurones. In our Leak / fast Na+
model, the existence of the rest state is largely due to the leak current IL, while the existence
of the excited state is largely due to the persistent inward Na+
current INa. Small
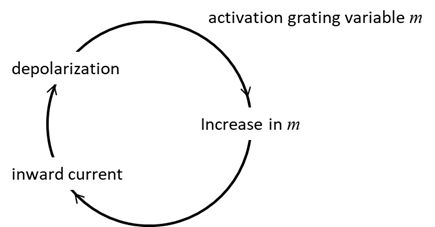
(sub-threshold) perturbations leave the state variable in the attraction
domain of the rest state, while large (super-threshold) perturbations
initiate the regenerative process where the upstroke of an action potential, and the voltage variable becomes attracted to
the excited state.
Fig.
17. Upstroke dynamics for the
generation of action potential for our Leak / fast Na+ model. The
model describes quite well the upstroke dynamics of layer 5 pyramidal
neurones. Iext = 0.60
mA
np002B.m Generation of the
action potential must be completed via repolarization that moves VM back to the rest state.
Typically, repolarization occurs because of a relatively slow inactivation of
Na+ current and/or slow activation of an outward K+
current, which are not taken into account our model. BIFURCATIONS A system is said to
undergo a bifurcation
when its qualitative behaviour changes. We can
investigate changing the external current injected into a neurone using our
leak / fast Na+ model. When the external
current is zero, all trajectories for initial membrane potentials are
attracted to the rest state where VM = - 67 mV
(figure 18).
Fig. 18. Iext = 0 mV There is only a single stable equilibrium point at the rest membrane potential VM = - 67
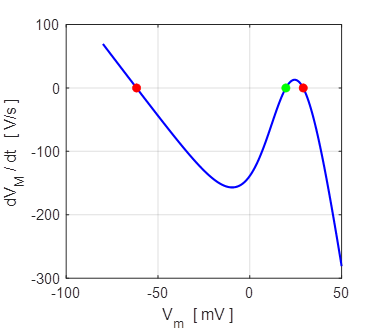
mV. Monostable np002B.m When the external current is increased Iext = 0.1 mA, then the system is bistable with stable
equilibrium at -62 mV (rest state) and + 29 mV (excited state) as shown in
figure 19.
Fig. 19. Iext = 0.1 mA There are two stable equilibrium points: rest membrane potential
-62 mV and the excited state +29 mV. The unstable
equilibrium point is around +20 mV.
Bistable
np002B.m When the external current is greater than
about 0.9 mA, there is again only one stable equilibrium
point as shown in figure 20.
Fig. 20 Iext = 0.9 mV There is only a single stable equilibrium point at the excited state
membrane potential
VM = 43
mV. Monostable
np002B.m We can clearly see that the qualitative
behaviour of our model depends upon the value of the external current. Iext = 0 mA, the system evolves to the resting
state. ~0.1 mA <
Iext < ~0.9 mA, the
system is bistable where the rest and excited states coexist. Iext > ~ 0.9 mA, the rest state no longer
exists because the leak current cannot cope with the large injected DC
injected current and the inward Na+ current. When Iext ~
0.9 mA a saddle-mode
bifurcation exists since
slight variations in Iex results in the system evolving to
a bistable or monstable state. As Iext is increased, the EXAMPLES The examples given are based upon the
Exercises in Chapter 3 of the Izhikevich’s
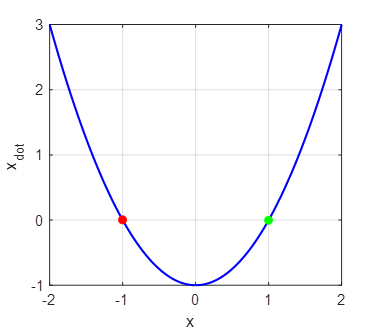
book. 1. Script np003.m System:
The red dot shows a stable equilibrium point and a green
dot an unstable
equilibrium point. For all initial values x < 1, the trajectory of the
system will evolve to the stable equilibrium point at x = -1. For all values of x > 1, the trajectory of the
system will diverge to infinity. The attraction domain corresponds to all
initial values x < 1. The eigenvalues are the roots of the function
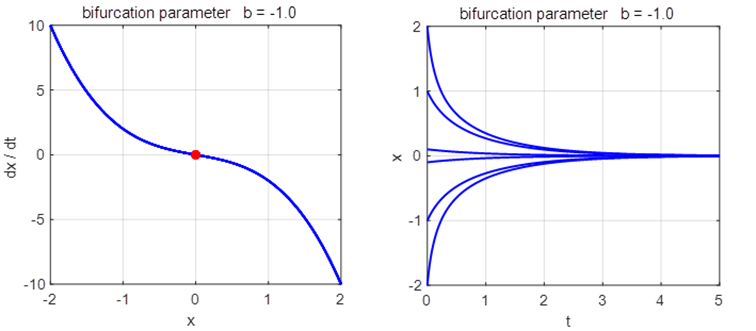
2. Script np003A.m System: The eigenvalues are
If If
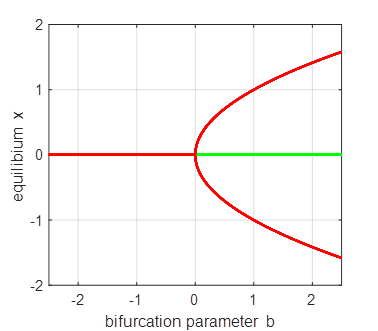
Saddle-node
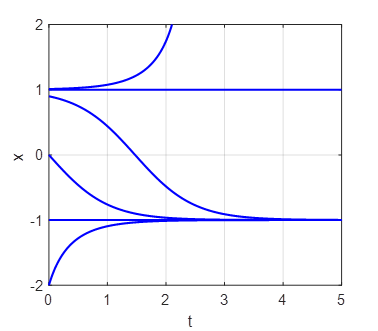
(fold) bifurcation diagram np003A.m The branch
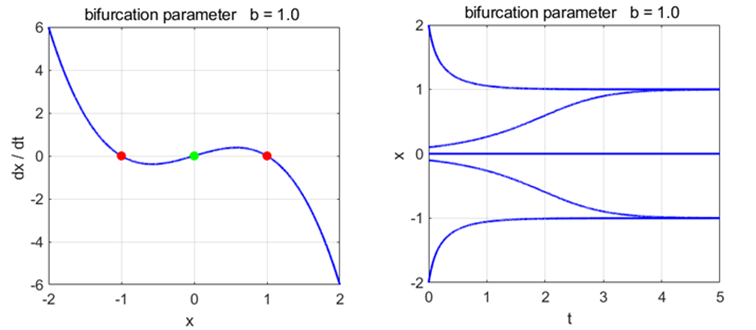
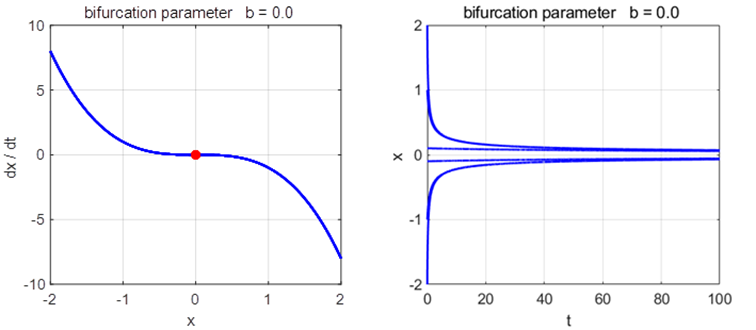
Phase
Portrait and Time Evolution Plots: b = +1, 0, and -1 np003A.m |